Nutrition and metabolism
... – is an important component of bile salts; formation of vitamin D, plasma membrane and steroid hormones – 15% from the diet - the rest is made from acetyl CoA in the liver, ...
... – is an important component of bile salts; formation of vitamin D, plasma membrane and steroid hormones – 15% from the diet - the rest is made from acetyl CoA in the liver, ...
Cellular Respiration
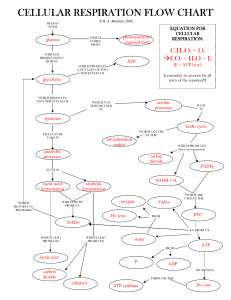
... Hydrogen carriers such as shuttle electrons in redox reactions Enzymes remove electrons from glucose molecules and transfer them to a coenzyme OXIDATION Dehydrogenase and NAD+ ...
... Hydrogen carriers such as shuttle electrons in redox reactions Enzymes remove electrons from glucose molecules and transfer them to a coenzyme OXIDATION Dehydrogenase and NAD+ ...
Tricarboxylic Acid Cycle
... production of eighteen ATP molecules, and the two molecules of FADH2 produce four ATP molecules, for a total of 22. The total is therefore the two ATP molecules produced directly plus the 22 molecules formed through the electron transport chain, which ...
... production of eighteen ATP molecules, and the two molecules of FADH2 produce four ATP molecules, for a total of 22. The total is therefore the two ATP molecules produced directly plus the 22 molecules formed through the electron transport chain, which ...
PowerPoint
... Transfers carbons to oxaloacetate (C4), forming citrate (C6) Cycles through steps to rearrange citrate 2 CO2 released Ends forming oxaloacetate Cycle starts again Net gain of 4 CO2, 6 NADH, 2 FADH2, 2 ATP ...
... Transfers carbons to oxaloacetate (C4), forming citrate (C6) Cycles through steps to rearrange citrate 2 CO2 released Ends forming oxaloacetate Cycle starts again Net gain of 4 CO2, 6 NADH, 2 FADH2, 2 ATP ...
Cellular Respiration
... NADH passes its 2 electrons to first H-acceptor and 2 H+ are pumped out to outer chamber (in between 2 membranes) of mitochondria. The remaining acceptors pump out two more H+ pairs to outer chamber by using energy of downhill moving electron pair. Therefore, 3 proton pairs are pumped by using the e ...
... NADH passes its 2 electrons to first H-acceptor and 2 H+ are pumped out to outer chamber (in between 2 membranes) of mitochondria. The remaining acceptors pump out two more H+ pairs to outer chamber by using energy of downhill moving electron pair. Therefore, 3 proton pairs are pumped by using the e ...
BI0 120 cell and tissues
... B. The proton gradient established during electron transport is a form of potential energy. C. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. D. The movement of protons down a concentration gradient is an endergonic process. E. ATP synthesis associ ...
... B. The proton gradient established during electron transport is a form of potential energy. C. The electron transport chain can be found in the mitochondria of aerobic bacteria and other cells. D. The movement of protons down a concentration gradient is an endergonic process. E. ATP synthesis associ ...
Honors Biology Notes:
... • ____________________: breakdown of macromolecules into monomers, releasing energy from the broken bonds • ____________________: building macromolecules from monomers, using energy to form bonds ATP (_________________________________________) powers cellular work – ATP loses one ___________________ ...
... • ____________________: breakdown of macromolecules into monomers, releasing energy from the broken bonds • ____________________: building macromolecules from monomers, using energy to form bonds ATP (_________________________________________) powers cellular work – ATP loses one ___________________ ...
Metabolism/Energy
... energy released as electrons move along, to pump H+ into the intermembrane space. This “coupling” of H+ flow and ATP synthesis is called chemiosmosis. The synthesis of ATP that results from the electron transport chain and chemiosmosis is referred to as oxidative phosphorylation. Oxidative phosphory ...
... energy released as electrons move along, to pump H+ into the intermembrane space. This “coupling” of H+ flow and ATP synthesis is called chemiosmosis. The synthesis of ATP that results from the electron transport chain and chemiosmosis is referred to as oxidative phosphorylation. Oxidative phosphory ...
Chapter 6 – How Cells Harvest Chemical Energy Standard 1.g
... 1. The movement of electrons along an electron transport chain creates a proton gradient across the inner membrane. The protons diffuse back across the membrane through ATP synthase releasing energy that is used to make ATP by 2. ATP can also be made by transferring phosphate groups from organic mol ...
... 1. The movement of electrons along an electron transport chain creates a proton gradient across the inner membrane. The protons diffuse back across the membrane through ATP synthase releasing energy that is used to make ATP by 2. ATP can also be made by transferring phosphate groups from organic mol ...
The Krebs Cycle (Citric Acid Cycle)
... Enzymes in the matrix of the mitochondria catalyze a cycle of reactions called the Krebs cycle. The common pathway to completely oxidize fuel molecules which mostly is acetyl CoA ,the product from the oxidative decarboxylation of pyruvate It enters the cycle and passes ten steps of reactions tha ...
... Enzymes in the matrix of the mitochondria catalyze a cycle of reactions called the Krebs cycle. The common pathway to completely oxidize fuel molecules which mostly is acetyl CoA ,the product from the oxidative decarboxylation of pyruvate It enters the cycle and passes ten steps of reactions tha ...
101 -- 2006
... __ 19. Which of these are attached to specific amino acids? a) ribosomal RNA c) messenger RNA e) RNA polymerase b) DNA d) transfer RNA __ 20. Has anticodons a) ribosomal RNA c) messenger RNA e) RNA polymerase b) DNA d) transfer RNA __ 21. Which occurs in the nucleus? a) Transcription only d) Transcr ...
... __ 19. Which of these are attached to specific amino acids? a) ribosomal RNA c) messenger RNA e) RNA polymerase b) DNA d) transfer RNA __ 20. Has anticodons a) ribosomal RNA c) messenger RNA e) RNA polymerase b) DNA d) transfer RNA __ 21. Which occurs in the nucleus? a) Transcription only d) Transcr ...
Practice Cellular Respiration Test
... b. enzymes make the reaction take place in small steps c. water prevents uncontrolled combustion d. no oxygen is needed e. no heat is released ...
... b. enzymes make the reaction take place in small steps c. water prevents uncontrolled combustion d. no oxygen is needed e. no heat is released ...
Cellular Respiration
... breakdown of compounds in the Krebs cycle is used to reduce NAD -> NADH and FAD -> FADH • NADH & FADH donate electrons to the ETC on the inner mitochondrial ...
... breakdown of compounds in the Krebs cycle is used to reduce NAD -> NADH and FAD -> FADH • NADH & FADH donate electrons to the ETC on the inner mitochondrial ...
Cell Respiration Power Point
... The Purpose of Cellular Respiration It is to make and break bonds to generate ATP and electrons. You end up with ATP, H ions and electrons. The electrons are sent to the Electron Transport Chain where they help to make ATP through ATP synthase. ****Hydrogen ions are bonded with oxygen to make water ...
... The Purpose of Cellular Respiration It is to make and break bonds to generate ATP and electrons. You end up with ATP, H ions and electrons. The electrons are sent to the Electron Transport Chain where they help to make ATP through ATP synthase. ****Hydrogen ions are bonded with oxygen to make water ...
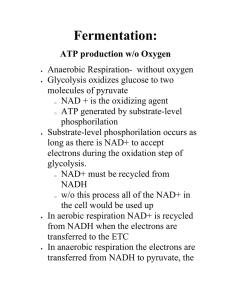
Chapter 9: Fermentation
... reduced directly by NADH to form lactate (ionized form of lactic acid). •Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt. •Muscle cells switch from aerobic respiration to lactic acid fermentation to generate ATP when O2 is scarce. •The waste product, lactate, ma ...
... reduced directly by NADH to form lactate (ionized form of lactic acid). •Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt. •Muscle cells switch from aerobic respiration to lactic acid fermentation to generate ATP when O2 is scarce. •The waste product, lactate, ma ...
Chapter 12 (part 1) - Nevada Agricultural Experiment
... • An oxidation involving FAD • Mechanism involves hydride removal by FAD and a deprotonation • This enzyme is actually part of the electron transport pathway in the inner mitochondrial membrane • The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) i ...
... • An oxidation involving FAD • Mechanism involves hydride removal by FAD and a deprotonation • This enzyme is actually part of the electron transport pathway in the inner mitochondrial membrane • The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) i ...
Biochem Midterm - Website of Neelay Gandhi
... 1. Which of the following statements concerning chemical reactions in the human body is incorrect? A. Due to the non-specific nature of enzymes, many reactions are able to be carried out in the human body that would otherwise proceed very slowly or not at all in nature. B. Cellular compartmentalizat ...
... 1. Which of the following statements concerning chemical reactions in the human body is incorrect? A. Due to the non-specific nature of enzymes, many reactions are able to be carried out in the human body that would otherwise proceed very slowly or not at all in nature. B. Cellular compartmentalizat ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.