Textbook sample chapter
... react together to form ‘compound atoms’. These later became known as molecules. Dalton studied the physical properties of air and gases. This led him to analytical work on ethene (olefiant gas), methane (carburetted hydrogen) and other gases. His atomic theory explained his chemical analyses. He sum ...
... react together to form ‘compound atoms’. These later became known as molecules. Dalton studied the physical properties of air and gases. This led him to analytical work on ethene (olefiant gas), methane (carburetted hydrogen) and other gases. His atomic theory explained his chemical analyses. He sum ...
0 Review Presentations
... ▸ “Atoms comes from Greek word “Atomos,”meaning “indivisible.” ▸ In 1897, J.J. Thompson created the Cathrode Ray Tube that discovered the ...
... ▸ “Atoms comes from Greek word “Atomos,”meaning “indivisible.” ▸ In 1897, J.J. Thompson created the Cathrode Ray Tube that discovered the ...
overview of semester 1
... In 1896, at roughly the same time as Thomson was working on his electrons, Henri Becquerel discovered the "radioactivity" of a uranium-bearing compound, pitchblende. We will discuss radioactivity in greater detail later. Around 1898 the French chemists Marie and Pierre Curie discovered two additiona ...
... In 1896, at roughly the same time as Thomson was working on his electrons, Henri Becquerel discovered the "radioactivity" of a uranium-bearing compound, pitchblende. We will discuss radioactivity in greater detail later. Around 1898 the French chemists Marie and Pierre Curie discovered two additiona ...
Elements Compounds
... Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. ...
... Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. ...
9.1 REDOX Introduction to Oxidation and Reduction
... copper and tin during the Bronze Age, 4500 BCE to 1200 BCE Copper & tin occur naturally, but this was the 1st evidence for the use of heat to reduce metallic ...
... copper and tin during the Bronze Age, 4500 BCE to 1200 BCE Copper & tin occur naturally, but this was the 1st evidence for the use of heat to reduce metallic ...
Unit 2: Atomic Concepts and Periodic Table (Level 1)
... o If your name has the letter J, use J and the information for Iodine (J was the former symbol for Iodine). o If you are only using the first letter of a two-lettered symbol, still include the second letter, but fade the letter so it does not appear that it is used in the spelling (see example at th ...
... o If your name has the letter J, use J and the information for Iodine (J was the former symbol for Iodine). o If you are only using the first letter of a two-lettered symbol, still include the second letter, but fade the letter so it does not appear that it is used in the spelling (see example at th ...
Atoms and the Periodic Table Atoms and the Periodic Table
... Metals shine because they are made of elements that reflect light. Another property of metals is that they do not shatter. Metals bend as they are pressed into thin, flat sheets during the coin-making process. All metals share some similarities, but each metal has its own unique chemical and physica ...
... Metals shine because they are made of elements that reflect light. Another property of metals is that they do not shatter. Metals bend as they are pressed into thin, flat sheets during the coin-making process. All metals share some similarities, but each metal has its own unique chemical and physica ...
This question is about the elements in Period 3 of the Periodic Table
... State the role of water in the reaction with calcium. ...
... State the role of water in the reaction with calcium. ...
View PDF - CiteSeerX
... the structure of the periodic system. Explicit references to the periodic system were rare in the 1870s, even in the chemistry literature, and only by the late 1880s had Mendeleev’s system become widely accepted and incorporated into most textbooks (Brush, 1996). In 1892 Thomson pointed out, for the ...
... the structure of the periodic system. Explicit references to the periodic system were rare in the 1870s, even in the chemistry literature, and only by the late 1880s had Mendeleev’s system become widely accepted and incorporated into most textbooks (Brush, 1996). In 1892 Thomson pointed out, for the ...
Chapter 4 Elements and the Periodic Table The Periodic Table
... Metals in the Periodic Table Group 2 of the periodic table contains the alkaline earth metals. These elements are not as reactive as the metals in Group 1, but they are more reactive than most other metals. ...
... Metals in the Periodic Table Group 2 of the periodic table contains the alkaline earth metals. These elements are not as reactive as the metals in Group 1, but they are more reactive than most other metals. ...
General and Inorganic Chemistry I.
... John Newlands was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into 11 groups which were based on similar physical properties. ...
... John Newlands was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into 11 groups which were based on similar physical properties. ...
Structure of the atom,english2009-08
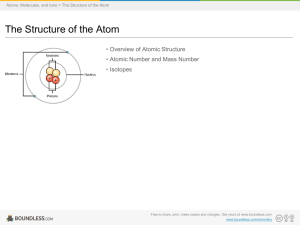
... After studying this chapter, students are expected tobe able to: Define the atom explain the development of the atomic theory/ model Describe the structure of atom containing subatomic particles : protons, neutrons, and electrons. Define atomic number (Z) and mass number (A) Define isotope ...
... After studying this chapter, students are expected tobe able to: Define the atom explain the development of the atomic theory/ model Describe the structure of atom containing subatomic particles : protons, neutrons, and electrons. Define atomic number (Z) and mass number (A) Define isotope ...
Chromatographic Enrichment of Lithium Isotopes by Hydrous
... lithium, 7Li could be used as a pH control agent (7LiOH) of the coolant in nuclear fission reactors. And the lithium compounds rich in 6Li will be required for the tritium breeder blanket in deuterium-tritium fusion power reactors in the future.7 Because of the large cross section for thermal neutro ...
... lithium, 7Li could be used as a pH control agent (7LiOH) of the coolant in nuclear fission reactors. And the lithium compounds rich in 6Li will be required for the tritium breeder blanket in deuterium-tritium fusion power reactors in the future.7 Because of the large cross section for thermal neutro ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... Determine the number of protons in a)Fe3+ b) P3• Fe is iron element #26. • Z = 26 = #p+s = #e-s in neutral atom • 3+ charge means 3 electrons lost 26 - 3 =23 e• P is phosphorus element #15. • Z = 15 = #p+s = #e-s in neutral atom • 3- charge means 3 electrons gained 15 + 3 =18 eTro's "Introductory C ...
... Determine the number of protons in a)Fe3+ b) P3• Fe is iron element #26. • Z = 26 = #p+s = #e-s in neutral atom • 3+ charge means 3 electrons lost 26 - 3 =23 e• P is phosphorus element #15. • Z = 15 = #p+s = #e-s in neutral atom • 3- charge means 3 electrons gained 15 + 3 =18 eTro's "Introductory C ...
Acquiring the Foundation: The Periodic Table for Middle
... animated periodic table of the first eighteen elements on the periodic table of elements. The animated periodic table of elements will be organized according to the same principles Mendeleyev used when he placed elements in the original periodic table. This unit will help students build a strong fou ...
... animated periodic table of the first eighteen elements on the periodic table of elements. The animated periodic table of elements will be organized according to the same principles Mendeleyev used when he placed elements in the original periodic table. This unit will help students build a strong fou ...
Nucleon number
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
Chapter 7 Periodic Properties of the Elements
... came to the same conclusion about how elements should be grouped. ...
... came to the same conclusion about how elements should be grouped. ...
9th class bridge course 74-112
... the radius and energy of an orbit. (iii) It could explain the atomic spectrum of hydrogen. • Spectrum: The group of wavelengths or frequencies is known as a spectrum. NARAYANA GROUP OF SCHOOLS ...
... the radius and energy of an orbit. (iii) It could explain the atomic spectrum of hydrogen. • Spectrum: The group of wavelengths or frequencies is known as a spectrum. NARAYANA GROUP OF SCHOOLS ...
PSN Chapter 13 Multi-format Test.tst
... 20. A bismuth atom which contains 83 protons and 127 neutrons decays to produce an atom of polonium with a mass number of 210 and 84 protons. What type of decay does bismuth experience? 21. Of the three sub-atomic particles: electrons, protons and neutrons, which determines most of the properties of ...
... 20. A bismuth atom which contains 83 protons and 127 neutrons decays to produce an atom of polonium with a mass number of 210 and 84 protons. What type of decay does bismuth experience? 21. Of the three sub-atomic particles: electrons, protons and neutrons, which determines most of the properties of ...
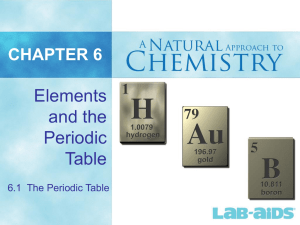
Elements and the Periodic Table
... The STM does not use light. Instead, it scans the surface of a sample with a tiny electric current to detect its shape. The image, including the colour, is then computer generated. Prior to the invention of STMs in 1981, no one had ever seen an image of an atom. Scientists could not have invented th ...
... The STM does not use light. Instead, it scans the surface of a sample with a tiny electric current to detect its shape. The image, including the colour, is then computer generated. Prior to the invention of STMs in 1981, no one had ever seen an image of an atom. Scientists could not have invented th ...
Balancing Reaction Equations Oxidation State Reduction
... change states in this step. Be sure the equation is balanced for both atoms and charge. • Write the net ionic equation by removing the spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
... change states in this step. Be sure the equation is balanced for both atoms and charge. • Write the net ionic equation by removing the spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge. ...
Atoms, Elements, and
... Models are often used for things that are too small or too large to be observed or that are too difficult to be understood easily. One way to make a model is to make a smaller version of something large. If you wanted to design a new sailboat, would you build a full-sized boat and hope it would floa ...
... Models are often used for things that are too small or too large to be observed or that are too difficult to be understood easily. One way to make a model is to make a smaller version of something large. If you wanted to design a new sailboat, would you build a full-sized boat and hope it would floa ...
Boundless Study Slides
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
Build an Atom Scripted - UTeach Outreach
... the table, so you don’t have to memorize a lot of information. Each element is given a symbol that represents its name, and is assigned a number called the atomic number. The atomic number tells you how many protons are in the nucleus of atoms of that element. Since the atomic number of carbon is 6, ...
... the table, so you don’t have to memorize a lot of information. Each element is given a symbol that represents its name, and is assigned a number called the atomic number. The atomic number tells you how many protons are in the nucleus of atoms of that element. Since the atomic number of carbon is 6, ...