FREE Sample Here
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
Chapter 04
... On the atomic mass scale for subatomic particles • 1 atomic mass unit (amu) is equal to 1/12 of the mass of the carbon-12 atom. • A proton has a mass of about 1 (1.007) amu. • A neutron has a mass of about 1 (1.008) amu. • An electron has a very small mass (0.00055 ...
... On the atomic mass scale for subatomic particles • 1 atomic mass unit (amu) is equal to 1/12 of the mass of the carbon-12 atom. • A proton has a mass of about 1 (1.007) amu. • A neutron has a mass of about 1 (1.008) amu. • An electron has a very small mass (0.00055 ...
FREE Sample Here
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
File
... Nitrogen because the electrons are closer to the nucleus since there are fewer energy levels – so the nucleus would be better able to hold onto an additional electron. ...
... Nitrogen because the electrons are closer to the nucleus since there are fewer energy levels – so the nucleus would be better able to hold onto an additional electron. ...
FREE Sample Here
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
... 4) What factors are most important in determining which elements are most common in living matter? A) the relative abundances of the elements in Earth's crust and atmosphere B) the emergent properties of the simple compounds made from these elements C) the reactivity of the elements with water D) th ...
PSN Chapter 14 Multi-format Test.tst
... repeated. This pattern is called ____________________ Short Answer 13. Two particles found in the nucleus of most atoms have masses equivalent to one atomic mass unit, or 1 amu. Name the particles. ...
... repeated. This pattern is called ____________________ Short Answer 13. Two particles found in the nucleus of most atoms have masses equivalent to one atomic mass unit, or 1 amu. Name the particles. ...
Preview Sample 1
... B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms. C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. D) outer-shell electrons of one atom are transferred to the inner electron shells of ...
... B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms. C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. D) outer-shell electrons of one atom are transferred to the inner electron shells of ...
Initial Pages.pmd - Sakshieducation.com
... In previous classes we learnt that elements were classified into metals and non-metals. But this classification had so many limitations. So, there was a need to classify them in other ways. Hence, chemists started to frame ways to group these elements and compounds on the basis of their physical and ...
... In previous classes we learnt that elements were classified into metals and non-metals. But this classification had so many limitations. So, there was a need to classify them in other ways. Hence, chemists started to frame ways to group these elements and compounds on the basis of their physical and ...
regents chemistry midterm - irondequoit 2014_entire exam w key
... Group 6) Many periodic trends can be observed on the modern periodic table. Answer the following questions about periodic trends on your answer sheet. a) Graph the periodic trend in atomic radius for Period 3 on the appropriate graph on your answer sheet. [1pt] b) Explain the cause for the observed ...
... Group 6) Many periodic trends can be observed on the modern periodic table. Answer the following questions about periodic trends on your answer sheet. a) Graph the periodic trend in atomic radius for Period 3 on the appropriate graph on your answer sheet. [1pt] b) Explain the cause for the observed ...
Introductory Chemistry, 2nd Edition Nivaldo Tro - Tutor
... very unreactive, practically inert very hard to remove electron from or give an electron to Tro's Introductory Chemistry, Chapter 4 ...
... very unreactive, practically inert very hard to remove electron from or give an electron to Tro's Introductory Chemistry, Chapter 4 ...
Periodic Classification of Elements
... 3. Which property of atoms was used by Mendeleev to classify the elements? 4. How many groups were originally proposed by Mendeleev in his periodic table? 5. Where in the periodic table are chemically similar elements placed, in a group or in a period? 6. Mendeleev’s periodic table had some blank sp ...
... 3. Which property of atoms was used by Mendeleev to classify the elements? 4. How many groups were originally proposed by Mendeleev in his periodic table? 5. Where in the periodic table are chemically similar elements placed, in a group or in a period? 6. Mendeleev’s periodic table had some blank sp ...
Topic 7b Redox notes
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
File
... more dense than water. Specific gravity has no units. It is simply a number. This is because the units cancel out when the densities of the two substances are compared. ...
... more dense than water. Specific gravity has no units. It is simply a number. This is because the units cancel out when the densities of the two substances are compared. ...
Atomic Structure
... By the late 1700s, chemists had accepted the definition of an element as a substance that cannot be broken down into simpler substances by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical pro ...
... By the late 1700s, chemists had accepted the definition of an element as a substance that cannot be broken down into simpler substances by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical pro ...
Oxidation
... Rules for Assigning Oxidation Numbers 1) The sum of the oxidation numbers will always equal the particle’s charge 2) The oxidation number for a neutral atom is always zero 3) Oxidation numbers for non–VOS metals depend on their group 4) Oxidation numbers for VOS metals are found based on anion 5) O ...
... Rules for Assigning Oxidation Numbers 1) The sum of the oxidation numbers will always equal the particle’s charge 2) The oxidation number for a neutral atom is always zero 3) Oxidation numbers for non–VOS metals depend on their group 4) Oxidation numbers for VOS metals are found based on anion 5) O ...
Question 2
... Many scientists have performed various experiments to verify the presence of charged particles in atoms. J.J. Thomson identified electrons (negatively charged particles), while E. Goldstein discovered protons (positively charged particles) present in atoms. As an atom is always neutral, the number o ...
... Many scientists have performed various experiments to verify the presence of charged particles in atoms. J.J. Thomson identified electrons (negatively charged particles), while E. Goldstein discovered protons (positively charged particles) present in atoms. As an atom is always neutral, the number o ...
Chapter 4 PowerPoint - Southeast Online
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
Book: The Structure of Atoms
... he Dalton theory of the atom and related ideas were the basis for our study of composition stoichiometry (Chapter 2) and reaction stoichiometry (Chapter 3), but that level of atomic theory leaves many questions unanswered. Why do atoms combine to form compounds? Why do they combine only in simple nu ...
... he Dalton theory of the atom and related ideas were the basis for our study of composition stoichiometry (Chapter 2) and reaction stoichiometry (Chapter 3), but that level of atomic theory leaves many questions unanswered. Why do atoms combine to form compounds? Why do they combine only in simple nu ...
The Atom
... nucleus electrons electron clouds OBJ ECTIVES ! Describe some of the experiments that led to the current atomic theory. ! Compare the different models of the atom. ! Explain how the atomic theory has changed as scientists have discovered new information about the atom. ...
... nucleus electrons electron clouds OBJ ECTIVES ! Describe some of the experiments that led to the current atomic theory. ! Compare the different models of the atom. ! Explain how the atomic theory has changed as scientists have discovered new information about the atom. ...
File
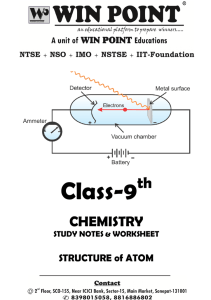
... (c) The electrons revolve rapidly round the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represented in two ways: either by the numbers 1, 2, 3, 4, 5 and 6 or by the letters K, L, M, N, O and P. The energy levels are counted from the centre outwards ...
... (c) The electrons revolve rapidly round the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represented in two ways: either by the numbers 1, 2, 3, 4, 5 and 6 or by the letters K, L, M, N, O and P. The energy levels are counted from the centre outwards ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
Inside the atom - Oxford University Press
... In the early 19th century, over a thousand years later, English chemist John Dalton built on Democritus’s idea of indivisible particles. He also suggested that different substances were made up of different particles that had specific masses and properties – elements. In other words, the particles t ...
... In the early 19th century, over a thousand years later, English chemist John Dalton built on Democritus’s idea of indivisible particles. He also suggested that different substances were made up of different particles that had specific masses and properties – elements. In other words, the particles t ...
elements of chemistry unit
... there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 oxidation number. The remaining two aluminum atoms must have + 3 oxidation numbers to balance out the oxygen oxidation numbers. Final oxidation numbers 4 Al(0) + 3 O2(-2) 2 Al2(+3)O3(-2) ...
... there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 oxidation number. The remaining two aluminum atoms must have + 3 oxidation numbers to balance out the oxygen oxidation numbers. Final oxidation numbers 4 Al(0) + 3 O2(-2) 2 Al2(+3)O3(-2) ...
classification of elements and periodicity in properties
... were incorrect. Mendeleev also had the foresight to leave gaps in the Periodic Table for elements unknown at that time and predict their properties from the trends that he observed among the properties of related elements. Mendeleev’s predictions were proved to be astonishingly correct when these el ...
... were incorrect. Mendeleev also had the foresight to leave gaps in the Periodic Table for elements unknown at that time and predict their properties from the trends that he observed among the properties of related elements. Mendeleev’s predictions were proved to be astonishingly correct when these el ...