FREE Sample Here
... Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of A) definite proportions. B) energy conservation. C) mass conservation. D) multiple proportions. Ans ...
... Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of A) definite proportions. B) energy conservation. C) mass conservation. D) multiple proportions. Ans ...
Chem I Review Part 2
... 92. Which of these substances will display an incomplete octet in its Lewis structure? A. CO2 B. Cl2 C. ICl D. NO E. SO2 93. Which of these elements is most likely to exhibit an expanded octet in its compounds? A. O B. S C. Na D. C E. N 94. According to the VSEPR theory, the shape of the SO3 molecu ...
... 92. Which of these substances will display an incomplete octet in its Lewis structure? A. CO2 B. Cl2 C. ICl D. NO E. SO2 93. Which of these elements is most likely to exhibit an expanded octet in its compounds? A. O B. S C. Na D. C E. N 94. According to the VSEPR theory, the shape of the SO3 molecu ...
General Chemistry: Atoms First (McMurry/Fay/Pribush)
... Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of A) definite proportions. B) energy conservation. C) mass conservation. D) multiple proportions. Ans ...
... Topic: Section 2.1 Conservation of Mass and the Law of Definite Proportions 2) The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of A) definite proportions. B) energy conservation. C) mass conservation. D) multiple proportions. Ans ...
9.2 Oxidation Numbers
... Are these reactions oxidation‑reduction reactions? Are electrons transferred? Simply reading a chemical equation does not always tell us whether oxidation and reduction have occurred, so chemists have developed a numerical system to help identify a reaction as redox. For redox reactions, this system ...
... Are these reactions oxidation‑reduction reactions? Are electrons transferred? Simply reading a chemical equation does not always tell us whether oxidation and reduction have occurred, so chemists have developed a numerical system to help identify a reaction as redox. For redox reactions, this system ...
A Chemical Progression
... we today call atoms was theoretically predicted thousands of years before there was any experimental evidence regarding them. Although Democritus’s resolution wasn’t necessarily true, since the paradox can be resolved mathematically, his idea still had merit. For the first time, people began thinkin ...
... we today call atoms was theoretically predicted thousands of years before there was any experimental evidence regarding them. Although Democritus’s resolution wasn’t necessarily true, since the paradox can be resolved mathematically, his idea still had merit. For the first time, people began thinkin ...
Elements and the Periodic Table
... Rutherford was surprised that a few particles were deflected strongly. This led him to propose an atomic model with a positively charged nucleus. ...
... Rutherford was surprised that a few particles were deflected strongly. This led him to propose an atomic model with a positively charged nucleus. ...
No Slide Title - MDC Faculty Home Pages
... Atomic Symbols are used to represent Isotopes Example: Isotopes of Magnesium ...
... Atomic Symbols are used to represent Isotopes Example: Isotopes of Magnesium ...
The Masses of Atoms
... of his pocket if he is a bit excited. What is remarkable is that he noticeably reduces in size as soon as he taken his third hand out of his pocket. His atomic radius is 0.76 Å then he has two arms, but with three arms it is only 0.64 Å! Mr. Manganese is one of Mr. Iron’s close friends, and they bot ...
... of his pocket if he is a bit excited. What is remarkable is that he noticeably reduces in size as soon as he taken his third hand out of his pocket. His atomic radius is 0.76 Å then he has two arms, but with three arms it is only 0.64 Å! Mr. Manganese is one of Mr. Iron’s close friends, and they bot ...
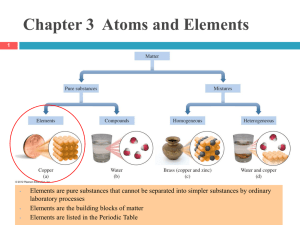
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
FREE Sample Here
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
FREE Sample Here
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
ChemistryReview
... correctly? Explain your answer. If the equation was not balanced correctly, write the correctly balanced equation. 77. How many moles of nitrogen are contained in 4.20 1024 atoms of nitrogen? 78. How many grams of O2 are in 5.0 mol of the element? ...
... correctly? Explain your answer. If the equation was not balanced correctly, write the correctly balanced equation. 77. How many moles of nitrogen are contained in 4.20 1024 atoms of nitrogen? 78. How many grams of O2 are in 5.0 mol of the element? ...
Chemistry
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
lesson plan - cloudfront.net
... as you watch, think about the battleship activity and try to make connections! 1. What charge were the particles that were shot out of the radioactive source? 2. What type of foil was put IN THE WAY of the beam of charged particles? 3. What happened to MOST of the particles? Did they bounce off or d ...
... as you watch, think about the battleship activity and try to make connections! 1. What charge were the particles that were shot out of the radioactive source? 2. What type of foil was put IN THE WAY of the beam of charged particles? 3. What happened to MOST of the particles? Did they bounce off or d ...
chapter - Grygla School
... Dalton’s theory explained most of the chemical data that existed during his time. As you will learn later in this chapter, data gathered since Dalton’s time shows that the first two principles are not true in all cases. Today, scientists can divide an atom into even smaller particles. Technology has ...
... Dalton’s theory explained most of the chemical data that existed during his time. As you will learn later in this chapter, data gathered since Dalton’s time shows that the first two principles are not true in all cases. Today, scientists can divide an atom into even smaller particles. Technology has ...
Has the Periodic Table Been Successfully Axiomatized?
... Soon afterwards, Thomson’s atomic model was deposed in favor of Rutherford’s nuclear atom in which the electrons were considered to orbit the small central nucleus. This task was achieved, partly, by Niels Bohr while on his postdoctoral year in Cambridge and Manchester following the completion of a ...
... Soon afterwards, Thomson’s atomic model was deposed in favor of Rutherford’s nuclear atom in which the electrons were considered to orbit the small central nucleus. This task was achieved, partly, by Niels Bohr while on his postdoctoral year in Cambridge and Manchester following the completion of a ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... chemical reactions, mass must be conserved. If elements could be changed into other elements during chemical reactions (as the alchemists were trying to do), then masses of atoms would change during reactions and mass would not be conserved. ...
... chemical reactions, mass must be conserved. If elements could be changed into other elements during chemical reactions (as the alchemists were trying to do), then masses of atoms would change during reactions and mass would not be conserved. ...
oxidation–reduction reaction
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 4 Elements and the Periodic Table
... reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
... reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
Chapter 4 Elements and the Periodic Table
... reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
... reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...