The Periodic Table
... A one-letter symbol, such as H or hydrogen, is capitalized. For a two-letter symbol, such as He for helium, only the first letter is capitalized. ...
... A one-letter symbol, such as H or hydrogen, is capitalized. For a two-letter symbol, such as He for helium, only the first letter is capitalized. ...
09 - Northern Highlands
... The periodic table is further divided into periods and groups. Each horizontal row is called a period. Across any period, the properties of the elements gradually change. Each vertical column is called a group. Groups of elements have similar properties. The main group elements are Groups 1-2 and 13 ...
... The periodic table is further divided into periods and groups. Each horizontal row is called a period. Across any period, the properties of the elements gradually change. Each vertical column is called a group. Groups of elements have similar properties. The main group elements are Groups 1-2 and 13 ...
Atomic Structure Powerpoints - Warren County Public Schools
... • Mass # = # of protons and # of neutrons in an atom. • Atomic Mass = The average mass for an element. It is determined by taking in account all the isotopes that make-up an element. You must know the isotopes relative abundance and mass # to calculate the atomic mass of the element. -Atomic mass = ...
... • Mass # = # of protons and # of neutrons in an atom. • Atomic Mass = The average mass for an element. It is determined by taking in account all the isotopes that make-up an element. You must know the isotopes relative abundance and mass # to calculate the atomic mass of the element. -Atomic mass = ...
EARLY ATOMIC THEORY AND STRUCTURE
... deflection. This shows that the atom is mostly empty space and the nucleus is very small. In Thomson’s experiments with the cathode ray tube, rays were observed coming from both the anode and the cathode. In Rutherford’s experiment an alpha particle was occasionally dramatically deflected by the nucle ...
... deflection. This shows that the atom is mostly empty space and the nucleus is very small. In Thomson’s experiments with the cathode ray tube, rays were observed coming from both the anode and the cathode. In Rutherford’s experiment an alpha particle was occasionally dramatically deflected by the nucle ...
AGS General Science Chapt 2
... The scanning electron microscope, or SEM, uses electron beams to look at very small items. The SEM makes a sharply detailed, 3-D picture. An SEM picture can show an item up to 200,000 times bigger than it is. The item is magnified so much that you can see molecules. ...
... The scanning electron microscope, or SEM, uses electron beams to look at very small items. The SEM makes a sharply detailed, 3-D picture. An SEM picture can show an item up to 200,000 times bigger than it is. The item is magnified so much that you can see molecules. ...
Study Guide for Final #1
... 1.) Know who the important contributors were who helped to derive the different models of the atom. Know what their contributions were. 2.) Be able to describe Dalton’s atomic theory. 3.) Know where the three different subatomic particles are located, their charges, and their relative sizes. 4.) Kno ...
... 1.) Know who the important contributors were who helped to derive the different models of the atom. Know what their contributions were. 2.) Be able to describe Dalton’s atomic theory. 3.) Know where the three different subatomic particles are located, their charges, and their relative sizes. 4.) Kno ...
Oxidation-Reduction (Redox) Reactions
... Identifying Redox Reactions First determine oxidation numbers of each species in the reaction and then identify the oxidation or reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain electrons (be reduced). B. Reducing Ag ...
... Identifying Redox Reactions First determine oxidation numbers of each species in the reaction and then identify the oxidation or reduction processes A. Oxidation and reduction occur together. Whenever an atom loses electrons (is oxidized) another atom must gain electrons (be reduced). B. Reducing Ag ...
3.1 The Element A. Abundances of Eleme B. Names and Symbols
... proteins, a group of substances that serve the human body in almost uncountable ways, are all made by linking together a few fundamental units to form huge molecules. A nonchemical example is the English language, in which hundreds of thousands of words are constructed from only 26 letters. Compoun ...
... proteins, a group of substances that serve the human body in almost uncountable ways, are all made by linking together a few fundamental units to form huge molecules. A nonchemical example is the English language, in which hundreds of thousands of words are constructed from only 26 letters. Compoun ...
1st block atomic structure ppts.
... Calculating Atomic Mass of an Element Atomic mass: • It is an average mass calculated from all the isotopes of a particular element. • The average mass is weighted because there is NOT an equal amount of each isotope in a sample. • How do you calculate a weighted average mass? 1. For each isotope, ...
... Calculating Atomic Mass of an Element Atomic mass: • It is an average mass calculated from all the isotopes of a particular element. • The average mass is weighted because there is NOT an equal amount of each isotope in a sample. • How do you calculate a weighted average mass? 1. For each isotope, ...
Atom
... such a way that there are patterns of elements placed close together that have similar properties. For example, knowing the properties of one element in a column of the periodic table will help a person predict the properties of other elements in that same column. –Describe two properties common to ...
... such a way that there are patterns of elements placed close together that have similar properties. For example, knowing the properties of one element in a column of the periodic table will help a person predict the properties of other elements in that same column. –Describe two properties common to ...
A Model of the Atom - Mrs. O`Hare Barrows` Classroom Web
... • Many scientists were not convinced that the cathode rays were streams of particles. • In 1897, J.J. Thomson, an English physicist, tried to clear up the confusion. • He placed a magnet beside the tube from ...
... • Many scientists were not convinced that the cathode rays were streams of particles. • In 1897, J.J. Thomson, an English physicist, tried to clear up the confusion. • He placed a magnet beside the tube from ...
Slide 1
... • Many scientists were not convinced that the cathode rays were streams of particles. • In 1897, J.J. Thomson, an English physicist, tried to clear up the confusion. • He placed a magnet beside the tube from ...
... • Many scientists were not convinced that the cathode rays were streams of particles. • In 1897, J.J. Thomson, an English physicist, tried to clear up the confusion. • He placed a magnet beside the tube from ...
EARLY ATOMIC THEORY AND STRUCTURE
... Dalton – no positive matter in his model Thomson – positive matter is distributed throughout the atom Rutherford – positive matter is concentrated in a small central nucleus ...
... Dalton – no positive matter in his model Thomson – positive matter is distributed throughout the atom Rutherford – positive matter is concentrated in a small central nucleus ...
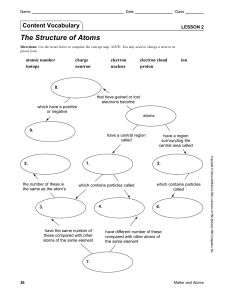
Lesson 2 | The Structure of Atoms
... 4. A negatively charged particle that occupies the space in an atom outside the nucleus is called a(n) electron. a. Electrons are much smaller in size than protons and neutrons, and they move very quickly. b. The region surrounding an atom’s nucleus, where one or more electrons are most likely to be ...
... 4. A negatively charged particle that occupies the space in an atom outside the nucleus is called a(n) electron. a. Electrons are much smaller in size than protons and neutrons, and they move very quickly. b. The region surrounding an atom’s nucleus, where one or more electrons are most likely to be ...
The Evolution of the Periodic System
... in London discovered the element argon; over the next few years, Ramsay announced the identification of four other elements—helium, neon, krypton and xenon—known as the noble gases. (The last of the known noble gases, radon, was discovered in 1900 by German physicist Friedrich Ernst Dorn.) The name ...
... in London discovered the element argon; over the next few years, Ramsay announced the identification of four other elements—helium, neon, krypton and xenon—known as the noble gases. (The last of the known noble gases, radon, was discovered in 1900 by German physicist Friedrich Ernst Dorn.) The name ...
The Evolution of the Periodic System - Science
... in London discovered the element argon; over the next few years, Ramsay announced the identification of four other elements—helium, neon, krypton and xenon—known as the noble gases. (The last of the known noble gases, radon, was discovered in 1900 by German physicist Friedrich Ernst Dorn.) The name ...
... in London discovered the element argon; over the next few years, Ramsay announced the identification of four other elements—helium, neon, krypton and xenon—known as the noble gases. (The last of the known noble gases, radon, was discovered in 1900 by German physicist Friedrich Ernst Dorn.) The name ...
GCSE Chemistry Textbook sample
... where different liquids with different boiling points are separated at different points in the process. Fractional distillation works by using a tall tower of gaps and surfaces, which are gradually colder towards the top. The liquid mixture is heated at the bottom and the liquids boil together to ...
... where different liquids with different boiling points are separated at different points in the process. Fractional distillation works by using a tall tower of gaps and surfaces, which are gradually colder towards the top. The liquid mixture is heated at the bottom and the liquids boil together to ...
Scheme of work
... This resource provides guidance for teaching the Atomic structure topic from our new GCSE in Combined Science: Trilogy/Physics. It has been updated from the draft version to reflect the changes made in the accredited specification such as the specification reference numbers. A few changes have also ...
... This resource provides guidance for teaching the Atomic structure topic from our new GCSE in Combined Science: Trilogy/Physics. It has been updated from the draft version to reflect the changes made in the accredited specification such as the specification reference numbers. A few changes have also ...
Name: Period:______ Table Number:______
... 51. Each element found on the periodic table of elements has a unique single letter (Hydrogen – H), two letter (Helium – He ) or three letter (Unnilquadiam – Unq) abbreviation which is called the CHEMICAL SYMBOL of that element. P. 83, Bill Nye the Science Guy Video 52. JONS BERZELIUS created the un ...
... 51. Each element found on the periodic table of elements has a unique single letter (Hydrogen – H), two letter (Helium – He ) or three letter (Unnilquadiam – Unq) abbreviation which is called the CHEMICAL SYMBOL of that element. P. 83, Bill Nye the Science Guy Video 52. JONS BERZELIUS created the un ...
Redox
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
... Before metallurgy, humans discovered fire. The technology of fire has been crucial in the development of human cultures, but only relatively recently (18th century) have we come to realize the role of oxygen in burning. Understanding the connection of corrosion (rusting, tarnishing, etc.) and burnin ...
GCSE Chemistry Textbook sample
... From understanding and preparing to teach new specifications, through to developing subject expertise and moving leadership, AQA has a training offering for you. Continued professional development training is provided to over 30,000 teachers each year, either through face to face, online or in-schoo ...
... From understanding and preparing to teach new specifications, through to developing subject expertise and moving leadership, AQA has a training offering for you. Continued professional development training is provided to over 30,000 teachers each year, either through face to face, online or in-schoo ...
Redox Reactions - Hillsborough County Public Schools
... That’s right, oxygen must be positive in this super rare case! ...
... That’s right, oxygen must be positive in this super rare case! ...
Gr 9 Atomic Structure_Gizmo Element Builder - OISE
... We have noted before that the outer shell of an atom has a special name, the valence shell. We also now that each orbital has a maximum number of electrons that it can accommodate. Most atoms prefer to have an outer shell/valence shell that is either full with the maximum number of electrons, or oth ...
... We have noted before that the outer shell of an atom has a special name, the valence shell. We also now that each orbital has a maximum number of electrons that it can accommodate. Most atoms prefer to have an outer shell/valence shell that is either full with the maximum number of electrons, or oth ...
Solutions-Manual-General-Organic-Biological
... In, Sn, Sb, Te, I and Xe are main group elements. ...
... In, Sn, Sb, Te, I and Xe are main group elements. ...