Chemistry UNIT 3 Test
... ____ 11. If three electrons are available to fill three empty 2p atomic orbitals, how will the electrons be distributed in the three orbitals? a. one electron in each orbital b. two electrons in one orbital, one in another, none in the third c. three in one orbital, none in the other two d. Three e ...
... ____ 11. If three electrons are available to fill three empty 2p atomic orbitals, how will the electrons be distributed in the three orbitals? a. one electron in each orbital b. two electrons in one orbital, one in another, none in the third c. three in one orbital, none in the other two d. Three e ...
atomic mass
... A) These reactions are called nuclear reactions, as they involve changes in the nucleus. ...
... A) These reactions are called nuclear reactions, as they involve changes in the nucleus. ...
Quantum-Mechanical Description of Mendeleev periodic table
... metallic gold film. If the model was correct, the massive α -particles (their mass is 7300 times that of the electron), would go through the film with minor deflections in their paths. The result was that many particles went just through but some particles were deflected at large angles [3]. Then he ...
... metallic gold film. If the model was correct, the massive α -particles (their mass is 7300 times that of the electron), would go through the film with minor deflections in their paths. The result was that many particles went just through but some particles were deflected at large angles [3]. Then he ...
MID-COURSE REVISION QUESTIONS The following questions are
... the bonding electrons are displaced closer to it. The covalent bond then has a slight ionic character and is said to be a polar covalent bond. (d) If when the individual polarities of all the bonds in a molecule are combined, an overall symmetry of the charge is not achieved, the molecule will have ...
... the bonding electrons are displaced closer to it. The covalent bond then has a slight ionic character and is said to be a polar covalent bond. (d) If when the individual polarities of all the bonds in a molecule are combined, an overall symmetry of the charge is not achieved, the molecule will have ...
MID-COURSE REVISION QUESTIONS The following questions are
... atoms allows each to obtain a share of two electrons via a covalent bond and an H 2 molecule results. As the overlapped orbits cannot contain any more electrons, it is not possible for a third or more H atom to join together to form H 3, H 4 etc. Question 2 Ionic bonds result from the complete trans ...
... atoms allows each to obtain a share of two electrons via a covalent bond and an H 2 molecule results. As the overlapped orbits cannot contain any more electrons, it is not possible for a third or more H atom to join together to form H 3, H 4 etc. Question 2 Ionic bonds result from the complete trans ...
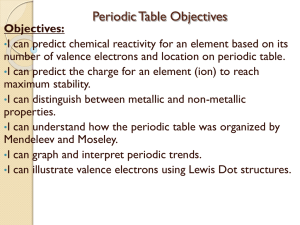
Using the Periodic Table
... • While the periodic table of elements looks confusing, it is actually very well organized – There are several patterns (called periodic trends) • For example: – The rows are called periods – The columns are called families ...
... • While the periodic table of elements looks confusing, it is actually very well organized – There are several patterns (called periodic trends) • For example: – The rows are called periods – The columns are called families ...
ATOMS
... THOMPSON’S & MILLIKAN’S IDEAS ATOMS ARE IN FACT DIVISIBLE. ELECTRONS ARE PRESENT IN ATOMS OF ALL ELEMENTS. ONE OF THE ATOM’S FUNDAMENTAL PARTICLES IS NEGATIVE CHARGED. ATOMS ARE ELECTRICALLY NEUTRAL, SO THERE MUST BE A (+) CHARGE TO BALANCE OUT THE (–). ...
... THOMPSON’S & MILLIKAN’S IDEAS ATOMS ARE IN FACT DIVISIBLE. ELECTRONS ARE PRESENT IN ATOMS OF ALL ELEMENTS. ONE OF THE ATOM’S FUNDAMENTAL PARTICLES IS NEGATIVE CHARGED. ATOMS ARE ELECTRICALLY NEUTRAL, SO THERE MUST BE A (+) CHARGE TO BALANCE OUT THE (–). ...
Chapter 11 section 2 questions - the atom
... Electrons are the negatively charged particles found in the energy levels that surround the nucleus - this like the rings on a target! Electrons have a VERY small mass - almost inconsequential to the atomic mass. Electrons carry a negative charge and are held in place by the positively charged proto ...
... Electrons are the negatively charged particles found in the energy levels that surround the nucleus - this like the rings on a target! Electrons have a VERY small mass - almost inconsequential to the atomic mass. Electrons carry a negative charge and are held in place by the positively charged proto ...
Quarterly 1 Review Trupia - Trupia
... c Explain why the electronegativity of elements in Group 17 decreases as you go down within that group. 85. A knowledge of the ionization energies of elements can be very useful in predicting the activity and type of reaction an element will have. a What does the ionization energy quantitatively mea ...
... c Explain why the electronegativity of elements in Group 17 decreases as you go down within that group. 85. A knowledge of the ionization energies of elements can be very useful in predicting the activity and type of reaction an element will have. a What does the ionization energy quantitatively mea ...
Electrons
... 4. The oxidation number of hydrogen is____except when it is bonded to metals in binary compounds. In these cases, its oxidation number is____. 5. Group 1 metals are____, Group 2 metals are____and fluorine is always____. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is eq ...
... 4. The oxidation number of hydrogen is____except when it is bonded to metals in binary compounds. In these cases, its oxidation number is____. 5. Group 1 metals are____, Group 2 metals are____and fluorine is always____. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is eq ...
Chapter 4 Presentation - Spearfish School District
... • He did this by measuring the deflection of helium nuclei by gold atoms. ...
... • He did this by measuring the deflection of helium nuclei by gold atoms. ...
Chapter 10 - MrsDoughertys
... Atoms are made up of protons, neutrons and electrons. Protons and neutrons have an electric charge. Electrons and protons are found in the nucleus of an atom. If you change the number of neutrons in the center of an atom, you change the type of ...
... Atoms are made up of protons, neutrons and electrons. Protons and neutrons have an electric charge. Electrons and protons are found in the nucleus of an atom. If you change the number of neutrons in the center of an atom, you change the type of ...
Structure of an Atom
... protons and neutrons. These e particles are known as subatomic particles. Electrons: The negatively charged particles in an atom are called electrons. An electron has one unit negative charge. The mass of an electron is 1/1837 of the mass of hydrogen atom (lightest atom). Thus, electrons have a negl ...
... protons and neutrons. These e particles are known as subatomic particles. Electrons: The negatively charged particles in an atom are called electrons. An electron has one unit negative charge. The mass of an electron is 1/1837 of the mass of hydrogen atom (lightest atom). Thus, electrons have a negl ...
2015 VCE Chemistry Unit 1 -Miss Fitzsimmons
... of the electron at the same time, which means you can't know the exact location of the electron. This principle proves error in Bohr's model because of the uncertainty of the location of an electron. ...
... of the electron at the same time, which means you can't know the exact location of the electron. This principle proves error in Bohr's model because of the uncertainty of the location of an electron. ...
Chemistry I Accelerated StudyGuideline
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...
Chapter 5
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
Modern Physics
... • An atom is stable (not radioactive) if it is in the belt of stability • An atom is unstable (radioactive) if it is outside the belt of stability • All elements beyond number 83, Bismuth are unstable - WHY? ...
... • An atom is stable (not radioactive) if it is in the belt of stability • An atom is unstable (radioactive) if it is outside the belt of stability • All elements beyond number 83, Bismuth are unstable - WHY? ...
Document
... source or how they were prepared, have the same proportions of their constituent elements. ...
... source or how they were prepared, have the same proportions of their constituent elements. ...
atoms. - Toolbox Pro
... • Fe has 4 orbitals • The first orbital closest to the nucleus contains 2 electrons. • The second contains 8. • The third contains 14. • The fourth contains 2 • Fe has 2 valence electrons (the # of electrons in the outermost shell) 2-8-14-2 is the Fe atom’s electron configuration ...
... • Fe has 4 orbitals • The first orbital closest to the nucleus contains 2 electrons. • The second contains 8. • The third contains 14. • The fourth contains 2 • Fe has 2 valence electrons (the # of electrons in the outermost shell) 2-8-14-2 is the Fe atom’s electron configuration ...
Objectives - Warren County Public Schools
... Bonus: What do chemist call electrons that occupy the highest energy level? ...
... Bonus: What do chemist call electrons that occupy the highest energy level? ...
Ch 6.7 - Explaining the Atom
... - An isotope is an atom with the same number of protons (3) for lithium, but a different Atomic Mass, 7u for Lithium. - Mass Number of 7 u – 3 (Protons) = 4 neutrons in the nucleus. - The True Atomic Mass of Lithium is not a whole number but a combination of Lithium 6u and Lithium 7u, mass number of ...
... - An isotope is an atom with the same number of protons (3) for lithium, but a different Atomic Mass, 7u for Lithium. - Mass Number of 7 u – 3 (Protons) = 4 neutrons in the nucleus. - The True Atomic Mass of Lithium is not a whole number but a combination of Lithium 6u and Lithium 7u, mass number of ...
Chapter 2 A particle view of matter
... multiplied unnecessarily’. Today, we would probably use the expression ‘keep it simple’. A more subtle interpretation of Ockham’s Razor would suggest the following: • If two competing theories have the very same predictions, then we adopt the simpler of the two theories. ...
... multiplied unnecessarily’. Today, we would probably use the expression ‘keep it simple’. A more subtle interpretation of Ockham’s Razor would suggest the following: • If two competing theories have the very same predictions, then we adopt the simpler of the two theories. ...