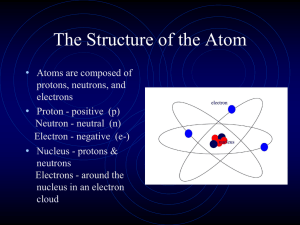
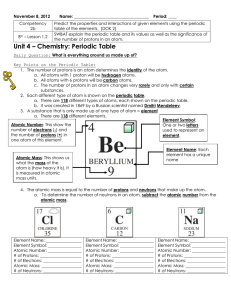
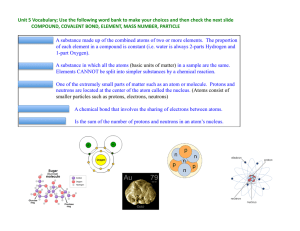
atom
... • Because gamma rays are massless, the emission of gamma rays by themselves cannot result in the formation of a new atom. ...
... • Because gamma rays are massless, the emission of gamma rays by themselves cannot result in the formation of a new atom. ...
PowerPoint
... • How can more than one form of atom make up an element? • What can be different and what is always the same among the different atoms of a given element? ...
... • How can more than one form of atom make up an element? • What can be different and what is always the same among the different atoms of a given element? ...
Atoms The configuration of subatomic particles within an
... Dalton’s ideas were far from perfect. However, they provided a basis for future experimentation to try and learn more about the stuff that makes up everything. J.J. Thomson was one such scientist who tried to add more detail to the model of the atom proposed by Dalton. ...
... Dalton’s ideas were far from perfect. However, they provided a basis for future experimentation to try and learn more about the stuff that makes up everything. J.J. Thomson was one such scientist who tried to add more detail to the model of the atom proposed by Dalton. ...
1 - Atomic Theory - Crestwood Local Schools
... void (makes change possible) • Elements differ in shape, position, and arrangement • Believed that atoms are: ...
... void (makes change possible) • Elements differ in shape, position, and arrangement • Believed that atoms are: ...
chapter-5-periodic-classification-of-elements
... Answer: No fixed position can be given to hydrogen in the Periodic Table. This was the first limitation of Mendeléev’s Periodic Table. He could not assign a correct position to hydrogen in his Table. Isotopes were discovered long after Mendeléev had proposed his periodic classification of elements. ...
... Answer: No fixed position can be given to hydrogen in the Periodic Table. This was the first limitation of Mendeléev’s Periodic Table. He could not assign a correct position to hydrogen in his Table. Isotopes were discovered long after Mendeléev had proposed his periodic classification of elements. ...
PPT
... Valence Shell : Outermost, highest energy shell of an atom. Valence electrons: An electron in an outermost shell of an atom. These electrons are loosely held, they are most important in determining an element’s properties. ...
... Valence Shell : Outermost, highest energy shell of an atom. Valence electrons: An electron in an outermost shell of an atom. These electrons are loosely held, they are most important in determining an element’s properties. ...
Chem Test 2 - TeacherWeb
... Identify the letter of the choice that best completes the statement or answers the question. ____ 21. Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson ____ 22. Which of the following was NOT among Democ ...
... Identify the letter of the choice that best completes the statement or answers the question. ____ 21. Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson ____ 22. Which of the following was NOT among Democ ...
1. The Greek philosopher Democritus coined what word for a tiny
... A. All elements are composed of atoms. B. All atoms of the same element have the same mass. C. Atoms contain subatomic particles. D. A compound contains atoms of more ...
... A. All elements are composed of atoms. B. All atoms of the same element have the same mass. C. Atoms contain subatomic particles. D. A compound contains atoms of more ...
Unit #3: ATOMIC STRUCTURE - Miss Virga`s Chemistry Class
... (1) They are positive subatomic particles and are found in the nucleus. (2) They are positive subatomic particles and are found surrounding the nucleus. (3) They are negative subatomic particles and are found in the nucleus. (4) They are negative subatomic particles and are found surrounding the nuc ...
... (1) They are positive subatomic particles and are found in the nucleus. (2) They are positive subatomic particles and are found surrounding the nucleus. (3) They are negative subatomic particles and are found in the nucleus. (4) They are negative subatomic particles and are found surrounding the nuc ...
6.7 Explaining the Periodic Table
... metal family, Be has two orbits, Mg has three orbits, and so on. • Within each family, all atoms have the same number of electrons in their outermost orbits. From the alkali metal family, Li has one outer electron, as do Na, K, and so on. This electron arrangement explains why the reaction of the a ...
... metal family, Be has two orbits, Mg has three orbits, and so on. • Within each family, all atoms have the same number of electrons in their outermost orbits. From the alkali metal family, Li has one outer electron, as do Na, K, and so on. This electron arrangement explains why the reaction of the a ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... 63 existing ELEMENTS in order by their increasing ATOMIC MASS number a. “PERIODIC” means having a regular, REPEATING pattern and it means a “listing” b. Mendeleev used the “periodic patterns” to PREDICT future ELEMENTS 4. In 1914, Moseley re-organized the PERIODIC Table according to each element’s ...
... 63 existing ELEMENTS in order by their increasing ATOMIC MASS number a. “PERIODIC” means having a regular, REPEATING pattern and it means a “listing” b. Mendeleev used the “periodic patterns” to PREDICT future ELEMENTS 4. In 1914, Moseley re-organized the PERIODIC Table according to each element’s ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
File
... 20. Element whose atoms lose electrons in chemical reactions to become positive ions. 21. Groups 3-12 on the periodic table. 22. Scientist who performed the gold foil experiment, and concluded that an atom must be composed of mostly empty space with a small, dense, positively-charged nucleus. 23. An ...
... 20. Element whose atoms lose electrons in chemical reactions to become positive ions. 21. Groups 3-12 on the periodic table. 22. Scientist who performed the gold foil experiment, and concluded that an atom must be composed of mostly empty space with a small, dense, positively-charged nucleus. 23. An ...
1.10 Atomic structure - Pearson Schools and FE Colleges
... All matter is made up of particles called atoms. Atoms are very, very small. It is not possible to see an atom even under the most powerful light microscope. However, scientists and engineers have developed ways of producing images of atoms using other methods. ...
... All matter is made up of particles called atoms. Atoms are very, very small. It is not possible to see an atom even under the most powerful light microscope. However, scientists and engineers have developed ways of producing images of atoms using other methods. ...
atomic - SandersScienceStuff
... 6. Bohr (1913)- suggested electrons must move around in well-defined orbits or energy levels a. His experiments suggested that electrons reside at different energy levels because it took more (or less) energy to knock them loose from an atom *Lets mark this for later: Bohr: planetary orbit of the el ...
... 6. Bohr (1913)- suggested electrons must move around in well-defined orbits or energy levels a. His experiments suggested that electrons reside at different energy levels because it took more (or less) energy to knock them loose from an atom *Lets mark this for later: Bohr: planetary orbit of the el ...
Atomic Model Stations - Moore Public Schools
... 6. Look over your data tables for protons, neutrons and electrons. Two things you notice are A. _ _____________________________________________ B. _ ____________________________________________________ 7. Put 3 protons into the nucleus of the atom. Then ...
... 6. Look over your data tables for protons, neutrons and electrons. Two things you notice are A. _ _____________________________________________ B. _ ____________________________________________________ 7. Put 3 protons into the nucleus of the atom. Then ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
Intro to the Periodic Table
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
Chemistry: Matter and Change
... • Because gamma rays are massless, the emission of gamma rays by themselves cannot result in the formation of a new atom. ...
... • Because gamma rays are massless, the emission of gamma rays by themselves cannot result in the formation of a new atom. ...
Periodic Table Notes
... • All neutral atoms have the same number electrons as protons. The atomic number tells us the number of protons, and it also tells us the total number of electrons. • The number of electrons surrounding the nucleus of an atom is equal to the number of protons in its nucleus. • How many electrons do ...
... • All neutral atoms have the same number electrons as protons. The atomic number tells us the number of protons, and it also tells us the total number of electrons. • The number of electrons surrounding the nucleus of an atom is equal to the number of protons in its nucleus. • How many electrons do ...
Review Unit 5
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
Atoms- Building Blocks TG quark.qxd
... There are different types of atoms with different numbers of protons, neutrons, and electrons. They are called elements. An electrically neutral atom has the same number of protons and electrons. Usually an atom has the same number of protons and neutrons but when it has more or less neutrons than p ...
... There are different types of atoms with different numbers of protons, neutrons, and electrons. They are called elements. An electrically neutral atom has the same number of protons and electrons. Usually an atom has the same number of protons and neutrons but when it has more or less neutrons than p ...
AP Chemistry Summer Assignment - Belle Vernon Area School District
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
Interactive Notebook 2 for 2011-2012
... All atoms of any given element have the same numbers of protons (atomic number = Z) in their nucleus. Atoms are identified based on the number protons in the nucleus. The Periodic Table is organized in order of increasing atomic number. However, atoms of the same element may have different numbers o ...
... All atoms of any given element have the same numbers of protons (atomic number = Z) in their nucleus. Atoms are identified based on the number protons in the nucleus. The Periodic Table is organized in order of increasing atomic number. However, atoms of the same element may have different numbers o ...