Document
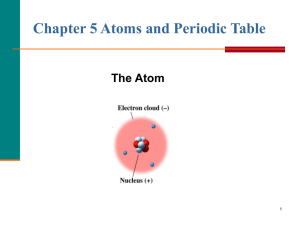
... 3. What does the Atomic Number tell you? 4. What does the Atomic Mass tell you? 5. What are the 3 parts of an atom? ...
... 3. What does the Atomic Number tell you? 4. What does the Atomic Mass tell you? 5. What are the 3 parts of an atom? ...
Chemistry
... o Be able to write numbers in scientific notation and standard form o Know the major units of measurement o Be able to identify the number of significant figures in a measurement o Be able to perform calculations using scientific notation and significant figures o Be able to correctly round a number ...
... o Be able to write numbers in scientific notation and standard form o Know the major units of measurement o Be able to identify the number of significant figures in a measurement o Be able to perform calculations using scientific notation and significant figures o Be able to correctly round a number ...
ATOMIC STRUacad test
... 14. Which of the following statements explains why chemists do not count atoms and molecules directly? A. Atoms and molecules are extremely small B. All of the relationships in a chemical reaction can be expressed as mass ratios C. Matter is neither created nor destroyed in a chemical reaction D. Re ...
... 14. Which of the following statements explains why chemists do not count atoms and molecules directly? A. Atoms and molecules are extremely small B. All of the relationships in a chemical reaction can be expressed as mass ratios C. Matter is neither created nor destroyed in a chemical reaction D. Re ...
Protons and Electrons
... atoms. Typically, atoms are stable when they have a full valence energy shell. This is referred to as the octet rule. Atoms with eight electrons in the valence shell are the most stable. This is true for most atoms except hydrogen and helium. These two elements have a full valence shell with two ele ...
... atoms. Typically, atoms are stable when they have a full valence energy shell. This is referred to as the octet rule. Atoms with eight electrons in the valence shell are the most stable. This is true for most atoms except hydrogen and helium. These two elements have a full valence shell with two ele ...
File
... 2) Made a mental model of the atom; Greek philosopher 3) Used by Rutherford in his experiment; made of two protons and two neutrons 4) The paths in which electrons circle the nucleus according to the Bohr model 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; ...
... 2) Made a mental model of the atom; Greek philosopher 3) Used by Rutherford in his experiment; made of two protons and two neutrons 4) The paths in which electrons circle the nucleus according to the Bohr model 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; ...
Document
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
Chapter 5
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
CHAPTER 4
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
... Example: Naturally occurring Cu consists of 2 isotopes. It is 69.1% 63Cu with a mass of 62.9 amu, and 30.9% 65Cu, which has a mass of 64.9 amu. Calculate the atomic weight of Cu to one decimal place. ...
Chapter 03 - La Salle University
... blocks, of elements according to the subshells that are last to fill, s, p, d, or f. ►Beginning at the top left corner of the periodic table, the first row contains only two elements, H and He. The 1s subshell is being filled here. ►The second row begins with two s-block elements (Li and Be) and con ...
... blocks, of elements according to the subshells that are last to fill, s, p, d, or f. ►Beginning at the top left corner of the periodic table, the first row contains only two elements, H and He. The 1s subshell is being filled here. ►The second row begins with two s-block elements (Li and Be) and con ...
File
... The Quantum Hypothesis • Max Planck, a German physicist, hypothesized that warm bodies emit radiant energy in discrete bundles called quanta. The energy in each energy bundle is proportional to the frequency of the radiation. • Einstein stated that light itself is quantized. A beam of light is not ...
... The Quantum Hypothesis • Max Planck, a German physicist, hypothesized that warm bodies emit radiant energy in discrete bundles called quanta. The energy in each energy bundle is proportional to the frequency of the radiation. • Einstein stated that light itself is quantized. A beam of light is not ...
Unit C3, C3.1
... Use the periodic table on the Data Sheet to answer these questions. The table below gives the electronic structures of four elements, W, X, Y and Z. ...
... Use the periodic table on the Data Sheet to answer these questions. The table below gives the electronic structures of four elements, W, X, Y and Z. ...
Chapter 4 Practice Test
... The particles that are found in the nucleus of an atom are ____. a. neutrons and electrons c. protons and neutrons b. electrons only d. protons and electrons As a consequence of the discovery of the nucleus by Rutherford, which model of the atom is thought to be true? a. Protons, electrons, and neut ...
... The particles that are found in the nucleus of an atom are ____. a. neutrons and electrons c. protons and neutrons b. electrons only d. protons and electrons As a consequence of the discovery of the nucleus by Rutherford, which model of the atom is thought to be true? a. Protons, electrons, and neut ...
Chemistry Study Guide
... ____ 57. Which step in the scientific method requires you to use your senses to obtain information? a. revising a hypothesis c. making an observation b. designing an experiment d. stating a theory ____ 58. The variable that is observed during an experiment is called what type of variable? a. indepe ...
... ____ 57. Which step in the scientific method requires you to use your senses to obtain information? a. revising a hypothesis c. making an observation b. designing an experiment d. stating a theory ____ 58. The variable that is observed during an experiment is called what type of variable? a. indepe ...
Chemistry Study Guide
... ____ 57. Which step in the scientific method requires you to use your senses to obtain information? a. revising a hypothesis c. making an observation b. designing an experiment d. stating a theory ____ 58. The variable that is observed during an experiment is called what type of variable? a. indepen ...
... ____ 57. Which step in the scientific method requires you to use your senses to obtain information? a. revising a hypothesis c. making an observation b. designing an experiment d. stating a theory ____ 58. The variable that is observed during an experiment is called what type of variable? a. indepen ...
Topic 2
... A greater than the mass of a neutron B the same as the mass of a proton C smaller than the mass of a proton D the same as the mass of a neutron (b) The atomic number of oxygen is 8. The mass number of an atom of oxygen is 17. Describe the number and type of particles in the nucleus of this atom. ...
... A greater than the mass of a neutron B the same as the mass of a proton C smaller than the mass of a proton D the same as the mass of a neutron (b) The atomic number of oxygen is 8. The mass number of an atom of oxygen is 17. Describe the number and type of particles in the nucleus of this atom. ...
The Atom and the Periodic Table
... I can describe how chemical properties of elements are similar in the same group. I can label each group by name (ex: alkali metals, halogens, noble gases, etc.) I can identify similar properties for each group. I can explain which groups are likely to react together ...
... I can describe how chemical properties of elements are similar in the same group. I can label each group by name (ex: alkali metals, halogens, noble gases, etc.) I can identify similar properties for each group. I can explain which groups are likely to react together ...
KS4 Atomic Structure 3747KB
... Elements consist of one type of atom, but sometimes these atoms can be slightly different. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. ...
... Elements consist of one type of atom, but sometimes these atoms can be slightly different. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. ...
Atomic Structure
... Elements consist of one type of atom, but sometimes these atoms can be slightly different. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. ...
... Elements consist of one type of atom, but sometimes these atoms can be slightly different. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. ...
KEY - Unit 3 Practice Qs
... An atom of oxygen has 8 protons and 8 electrons. 17. Explain, in terms of subatomic particles, why an oxide ion, O2-, has a negative charge. An oxide ion has 2 more electrons than protons, giving it a charge of -2. 18. Compare the number of protons to the number of electrons in a positive ion. A pos ...
... An atom of oxygen has 8 protons and 8 electrons. 17. Explain, in terms of subatomic particles, why an oxide ion, O2-, has a negative charge. An oxide ion has 2 more electrons than protons, giving it a charge of -2. 18. Compare the number of protons to the number of electrons in a positive ion. A pos ...
sample
... iron, have been known for thousands of years. Others have been discovered much more recently. Helium, often used in balloons, was discovered in 1895. Americium, used in smoke alarms, was discovered only in 1944. Scientists continue to discover new elements today. The atomic number (proton number) of ...
... iron, have been known for thousands of years. Others have been discovered much more recently. Helium, often used in balloons, was discovered in 1895. Americium, used in smoke alarms, was discovered only in 1944. Scientists continue to discover new elements today. The atomic number (proton number) of ...
(a) Atoms - Warren County Public Schools
... first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven. ...
... first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven. ...
Symbols of Elements - Chemistry with Mr. Patmos
... Isotopes of Magnesium In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
... Isotopes of Magnesium In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
PowerPoint
... • How can more than one form of atom make up an element? • What can be different and what is always the same among the different atoms of a given element? ...
... • How can more than one form of atom make up an element? • What can be different and what is always the same among the different atoms of a given element? ...
chemistry - Illini West High School
... group trends of several properties. • Relate period and group trends in atomic radii to electron configuration. ...
... group trends of several properties. • Relate period and group trends in atomic radii to electron configuration. ...