Page 1 of 9 Chem 103 Practice Problems: Below is a key for both
... Solution: Similar to the problem above. So, vrx = (1/2)(3.0x10-2)=1.5x10-2mol/min ii) the rate at which ammonium nitrate is used up. Solution: Since vrxn = (1/2)(dNH4NO3/dt) we can write: dNH4NO3/dt = 2 vrxn = 2(1.5x10-2mol/min) = 3.0x10-2mol/min iii) the rate at which oxygen is formed. Since vrxn = ...
... Solution: Similar to the problem above. So, vrx = (1/2)(3.0x10-2)=1.5x10-2mol/min ii) the rate at which ammonium nitrate is used up. Solution: Since vrxn = (1/2)(dNH4NO3/dt) we can write: dNH4NO3/dt = 2 vrxn = 2(1.5x10-2mol/min) = 3.0x10-2mol/min iii) the rate at which oxygen is formed. Since vrxn = ...
fahad h. ahmad - Fahad`s Academy
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
8 SHS Ch 8 Lecture shs_ch_8_lecture_2012
... Net Ionic Equations Equations for precipitation reactions (3): Molecular Equations All reactants and products are written as if they are molecules Ionic Equations All reactants and products that are soluble are written as ions, only the precipitate is written as if it were a molecule Net Ionic Equat ...
... Net Ionic Equations Equations for precipitation reactions (3): Molecular Equations All reactants and products are written as if they are molecules Ionic Equations All reactants and products that are soluble are written as ions, only the precipitate is written as if it were a molecule Net Ionic Equat ...
ANALYSIS OF THE SILVER GROUP CATIONS
... separate the mixture into subgroups that consist of just a few ions. Then it may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test ...
... separate the mixture into subgroups that consist of just a few ions. Then it may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test ...
Part II - American Chemical Society
... d. Solutions of lead acetate and sulfuric acid are mixed. e. Excess concentrated sodium hydroxide is added to a solution of zinc nitrate. f. Fluorine-18 undergoes positron emission. ...
... d. Solutions of lead acetate and sulfuric acid are mixed. e. Excess concentrated sodium hydroxide is added to a solution of zinc nitrate. f. Fluorine-18 undergoes positron emission. ...
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
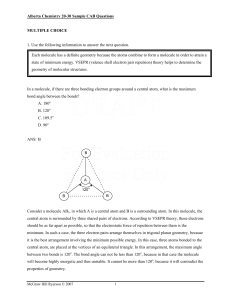
... should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geometry, because it is the best arrangement involving the minimum possible energy. In this case, three atoms b ...
... should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geometry, because it is the best arrangement involving the minimum possible energy. In this case, three atoms b ...
The Chemistry of Solutions Page | 1 Unit 7: The Chemistry of
... Dissociation/ Dissolution/ Solvation Equations When a soluble ionic compound is placed in water the ionic substance or salt will dissociate into its component ions. Ex. SrCl2 ...
... Dissociation/ Dissolution/ Solvation Equations When a soluble ionic compound is placed in water the ionic substance or salt will dissociate into its component ions. Ex. SrCl2 ...
Singlet Oxygen Production by Soybean Lipoxygenase Isozymes”
... scattered light toward the detector as well as the relatively long wavelength of light tended to minimize the reduction in chemiluminescence caused by turbidity. For example, the addition of 500 PM linoleic acid and 500 PM hydroperoxylinoleic acid to p2H 5, 50 mM sodium acetate resulted in a turbid ...
... scattered light toward the detector as well as the relatively long wavelength of light tended to minimize the reduction in chemiluminescence caused by turbidity. For example, the addition of 500 PM linoleic acid and 500 PM hydroperoxylinoleic acid to p2H 5, 50 mM sodium acetate resulted in a turbid ...
CD Roms - 香港道教聯合會圓玄學院第一中學
... Please use [CM] to denote ‘moral and civic education’ and [VA] to denote ‘value/attitude education’ in the Column of Learning Objective Column. 在活動/實驗/教具欄目內,註明合作學習、透過閱讀進行學習、英文增潤學習(例如教授英文詞彙等),以﹝CO﹞代表合作學習,﹝RL﹞代表透過閱讀進行學習,﹝EL﹞代表英文增潤學習。 Please use [CO] to denote ‘cooperative learning’, [RL] to denote ‘re ...
... Please use [CM] to denote ‘moral and civic education’ and [VA] to denote ‘value/attitude education’ in the Column of Learning Objective Column. 在活動/實驗/教具欄目內,註明合作學習、透過閱讀進行學習、英文增潤學習(例如教授英文詞彙等),以﹝CO﹞代表合作學習,﹝RL﹞代表透過閱讀進行學習,﹝EL﹞代表英文增潤學習。 Please use [CO] to denote ‘cooperative learning’, [RL] to denote ‘re ...
How to Use Reaction Stoichiometry
... Figure 4.6 (a) When an octane molecule undergoes complete combustion, it forms carbon dioxide and water: one CO2 molecule is formed for each carbon atom present (yellow arrows). (b) However, in a limited supply of oxygen, some of the carbon atoms end up as carbon monoxide molecules, CO, so the yiel ...
... Figure 4.6 (a) When an octane molecule undergoes complete combustion, it forms carbon dioxide and water: one CO2 molecule is formed for each carbon atom present (yellow arrows). (b) However, in a limited supply of oxygen, some of the carbon atoms end up as carbon monoxide molecules, CO, so the yiel ...
CHAPtER 9 Properties and reactions of organic compounds
... atoms bonded to one of the three highly electronegative elements F, O and N, and another molecule that must also contain an electronegative atom, such as oxygen and nitrogen. Hydrogen bonds are stronger intermolecular forces than both dispersion forces and dipole–dipole attractions. Hydrogen bonding ...
... atoms bonded to one of the three highly electronegative elements F, O and N, and another molecule that must also contain an electronegative atom, such as oxygen and nitrogen. Hydrogen bonds are stronger intermolecular forces than both dispersion forces and dipole–dipole attractions. Hydrogen bonding ...
Chapter 18.2
... Equations [19.1] • (Note: This section is usually covered with a very brief review of oxidation numbers and oxidizing/reducing agents, as covered in Chemistry 1010, which is found in section 4.9 of the Tro text). ...
... Equations [19.1] • (Note: This section is usually covered with a very brief review of oxidation numbers and oxidizing/reducing agents, as covered in Chemistry 1010, which is found in section 4.9 of the Tro text). ...
www.fahadsacademy.com
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
B - eko.olunet.org
... a) Write balanced ionic equations for aforementioned reactions. b) Calculate i) the amount of excess AgNO3, ii) mass of KBr in the resulting solution, iii) purity of KBr in the sample (in per cent), assuming that there are no traces of other halides in the technical grade KBr. c) Can Thomas use the ...
... a) Write balanced ionic equations for aforementioned reactions. b) Calculate i) the amount of excess AgNO3, ii) mass of KBr in the resulting solution, iii) purity of KBr in the sample (in per cent), assuming that there are no traces of other halides in the technical grade KBr. c) Can Thomas use the ...
TDB-5: Standards and conventions for TDB publications
... The same chemical formula may refer to different chemical species and must often be specified more clearly in order to avoid ambiguities. For example, UF 4 occurs as a gas, a solid, and an aqueous complex. The distinction between the different phases is made by phase designators that immediately fol ...
... The same chemical formula may refer to different chemical species and must often be specified more clearly in order to avoid ambiguities. For example, UF 4 occurs as a gas, a solid, and an aqueous complex. The distinction between the different phases is made by phase designators that immediately fol ...
SCH4U Exam Review
... 10. When 14 g of CO(g) and 9 g of H2O(g) are placed in a 5.0 L container, the following reaction occurs: CO(g) + H2O(g) H2 (g) + CO2 (g) At equilibrium there are 16.33g of CO2. What is the value of the equilibrium constant? ANS: 8.3 11. The reaction H2 (g) + Br2 (g) 2HBr(g) has an equilibrium co ...
... 10. When 14 g of CO(g) and 9 g of H2O(g) are placed in a 5.0 L container, the following reaction occurs: CO(g) + H2O(g) H2 (g) + CO2 (g) At equilibrium there are 16.33g of CO2. What is the value of the equilibrium constant? ANS: 8.3 11. The reaction H2 (g) + Br2 (g) 2HBr(g) has an equilibrium co ...
chromatographic study of photolysis of aqueous cyanocobalamin
... with those of the authentic compounds in specific solvent systems given under thin-layer chromatography. Hydroxocobalamin (solvent systems S1 and S2) was found to be the only photoproduct of cyanocobalamin at pH 1-7 alone, or in the presence of individual B/C vitamins. The relative intensity of the ...
... with those of the authentic compounds in specific solvent systems given under thin-layer chromatography. Hydroxocobalamin (solvent systems S1 and S2) was found to be the only photoproduct of cyanocobalamin at pH 1-7 alone, or in the presence of individual B/C vitamins. The relative intensity of the ...
Document
... condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium constant is a dimensionless quantity. 4. In quoting ...
... condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium constant is a dimensionless quantity. 4. In quoting ...
380 KB / 39 pages
... chemical species. (d) When a glass of ice cubes and water is left overnight and there are no ice cubes in the water the next morning, no chemical reaction has occurred. The ice cubes melted, H2O(s) → H2O(l), but no new chemical species formed. Problem 6.2. (a) When a match is dropped on the floor, n ...
... chemical species. (d) When a glass of ice cubes and water is left overnight and there are no ice cubes in the water the next morning, no chemical reaction has occurred. The ice cubes melted, H2O(s) → H2O(l), but no new chemical species formed. Problem 6.2. (a) When a match is dropped on the floor, n ...
www.XtremePapers.com
... For each of the questions in this section, one or more of the three numbered statements 1 to 3 may be correct. Decide whether each of the statements is or is not correct (you may find it helpful to put a tick against the statements that you consider to be correct). The responses A to D should be sel ...
... For each of the questions in this section, one or more of the three numbered statements 1 to 3 may be correct. Decide whether each of the statements is or is not correct (you may find it helpful to put a tick against the statements that you consider to be correct). The responses A to D should be sel ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO 3)2 (calcium nitrate), C6H12O6 (glucose), NaC2H3O2 (sodium acetate), and HC2H3O2 (acetic acid). Rank the solutions in order of increasing electrical conductivity, based on the fact that the gre ...
... Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO 3)2 (calcium nitrate), C6H12O6 (glucose), NaC2H3O2 (sodium acetate), and HC2H3O2 (acetic acid). Rank the solutions in order of increasing electrical conductivity, based on the fact that the gre ...