Chapters 14 and 15 Outline
... pH meter – is a device that determines the pH of a solution by measuring the voltage between the two electrodes that are in the solution. Titration – is a controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measure of a solution ...
... pH meter – is a device that determines the pH of a solution by measuring the voltage between the two electrodes that are in the solution. Titration – is a controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measure of a solution ...
Chemistry: Graphing Exercise
... A beaker containing 100 ml of water is heated at a constant rate for 10 minutes. The temperature of the liquid is recorded each minute. A second beaker containing 100 ml of ethanoic acid is heated and monitored under similar conditions. The data are recorded below. ...
... A beaker containing 100 ml of water is heated at a constant rate for 10 minutes. The temperature of the liquid is recorded each minute. A second beaker containing 100 ml of ethanoic acid is heated and monitored under similar conditions. The data are recorded below. ...
Chemistry Honors: Lesson 6 Acids and Bases Definitions 1
... Bronsted-Lowry base accepts protons. However, they can not be called Arrhenius bases since in aqueous solution they do not dissociate to form OH-. The advantage of this definition is that it is not limited to aqueous solutions. Bronsted-Lowry acids and bases always occur in pairs called conjugate ac ...
... Bronsted-Lowry base accepts protons. However, they can not be called Arrhenius bases since in aqueous solution they do not dissociate to form OH-. The advantage of this definition is that it is not limited to aqueous solutions. Bronsted-Lowry acids and bases always occur in pairs called conjugate ac ...
Quiz 1
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
A buffer solution is one that will maintain a rather constant pH value
... base is added to the solution. A very common buffer solution is blood which maintains its pH at about 7.4, “physiological pH.” ...
... base is added to the solution. A very common buffer solution is blood which maintains its pH at about 7.4, “physiological pH.” ...
K b
... bases are added or when dilution occurs. • The buffer is a mixture of an acid and its conjugate base. There must be comparable amounts of the conjugate acid and base (say, within a factor of 10) to exert significant buffering. ...
... bases are added or when dilution occurs. • The buffer is a mixture of an acid and its conjugate base. There must be comparable amounts of the conjugate acid and base (say, within a factor of 10) to exert significant buffering. ...
Calculating a Ka Value from a Known pH - Chemwiki
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
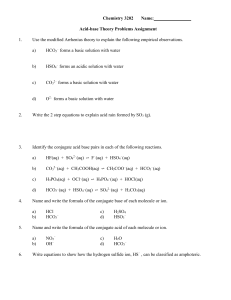
Chemistry 3202 Name: Acid-base Theory Problems Assignment 1
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
acids: bases - IDS-chem2-Rn-10
... a salt and water. A salt is any ionic compound that could be made with the anion of an acid ...
... a salt and water. A salt is any ionic compound that could be made with the anion of an acid ...
5 - Polyprotic Acids
... Subsequent dissociations will be ________________ by hydronium _____________ from the first dissociation ...
... Subsequent dissociations will be ________________ by hydronium _____________ from the first dissociation ...