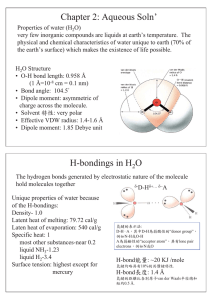
Review Sheet Exam 2 3.4-4.7
... How many grams of aspirin would you obtain from 4.5grams of salicylic acid with a percent yield of 75%? C7H6O3+ C4H6O3C9H8O4+C2H4O2 Salicylic acetic aspirin acetic Acid acid acid ...
... How many grams of aspirin would you obtain from 4.5grams of salicylic acid with a percent yield of 75%? C7H6O3+ C4H6O3C9H8O4+C2H4O2 Salicylic acetic aspirin acetic Acid acid acid ...
Advanced Placement Chemistry: 1984 Free Response Questions
... 7) The van der Waals equation of state for one mole of a real gas is as follows: (P + a/V2) (V - b) = RT For any given gas, the values of the constants a and b can be determined experimentally. Indicate which physical properties of a molecule determine the magnitudes of the constants a and b . Which ...
... 7) The van der Waals equation of state for one mole of a real gas is as follows: (P + a/V2) (V - b) = RT For any given gas, the values of the constants a and b can be determined experimentally. Indicate which physical properties of a molecule determine the magnitudes of the constants a and b . Which ...
1 - contentextra
... pH meter A device used to measure the pH of a solution. It can be analogue, digital or a data logging device, and can also be calibrated to read conductivity. pH scale A convenient means of expressing and comparing the hydrogen ion concentration of solutions. It is defined as –log [H+]. Spectator io ...
... pH meter A device used to measure the pH of a solution. It can be analogue, digital or a data logging device, and can also be calibrated to read conductivity. pH scale A convenient means of expressing and comparing the hydrogen ion concentration of solutions. It is defined as –log [H+]. Spectator io ...
Types of Weathering
... Dissolution Rocks, when in water, react with acids in the water and dissolve. A clue that this has happened to a rock is the presence of small holes. ...
... Dissolution Rocks, when in water, react with acids in the water and dissolve. A clue that this has happened to a rock is the presence of small holes. ...
Weak Acids and Bases Practice -- Chemistry 121A
... yield. It runs from 0% (all reactants) to 100% (all products). This is simply more convenient than Q, which runs from 0 to infinity. The way I look at it is this for a weak acid: %ionization = [A−]eq/[HA]o x 100% That is, the “final” concentration of A− at equilibrium divided by the “initial” concen ...
... yield. It runs from 0% (all reactants) to 100% (all products). This is simply more convenient than Q, which runs from 0 to infinity. The way I look at it is this for a weak acid: %ionization = [A−]eq/[HA]o x 100% That is, the “final” concentration of A− at equilibrium divided by the “initial” concen ...
Titration Worksheet
... of the solution. C. Calculate the molar mass of the unknown acid. B. Calculate the moles of in the ...
... of the solution. C. Calculate the molar mass of the unknown acid. B. Calculate the moles of in the ...
Topic 8.4 Acids and Bases The pH Scale
... Adding a Base……….. If we add more OH- what is going to happen to the ...
... Adding a Base……….. If we add more OH- what is going to happen to the ...
PRACTICE TEST for EXAM 10
... the pH of a solution if the concentration of hydronium ion increases? What if it decreases? 7. Compare and contrast a strong acid and a weak acid, in terms of neutralization titration results, pH, and conductivity, and explain their differences in terms of their reaction with water. 8. A weak acid i ...
... the pH of a solution if the concentration of hydronium ion increases? What if it decreases? 7. Compare and contrast a strong acid and a weak acid, in terms of neutralization titration results, pH, and conductivity, and explain their differences in terms of their reaction with water. 8. A weak acid i ...
Acids and Bases - Parkway C-2
... 3. Which of these solutions is the most basic? a. [H3O ] = 1 10 M c. [H3O ] = 1 10 M b. [OH ] = 1 10 M d. [OH ] = 1 10 M 4. An indicator is what type of compound? a. oxidizing agent c. strong base or acid b. weak base or acid d. salt 5. What characterizes a strong acid or base? a. polar covalent bon ...
... 3. Which of these solutions is the most basic? a. [H3O ] = 1 10 M c. [H3O ] = 1 10 M b. [OH ] = 1 10 M d. [OH ] = 1 10 M 4. An indicator is what type of compound? a. oxidizing agent c. strong base or acid b. weak base or acid d. salt 5. What characterizes a strong acid or base? a. polar covalent bon ...
Unit Seven Worksheet – 2
... Ratio of the concentration of the dissociated (or ionized) form of an acid to the concentration of the undissociated acid; symbolized Ka Base that dissociates completely into metal ions and hydroxide ions in aqueous solution Acid that completely ionizes in aqueous solution Base that does not dissoci ...
... Ratio of the concentration of the dissociated (or ionized) form of an acid to the concentration of the undissociated acid; symbolized Ka Base that dissociates completely into metal ions and hydroxide ions in aqueous solution Acid that completely ionizes in aqueous solution Base that does not dissoci ...
CHEM121 Exam 4 ObjectivesW16
... Calculate equilibrium constants (given concentrations of reactants/products) Interpret equilibrium constants (large/small are products/reactants favored?) LeChatelier’s Principle -shift Left or Right in response to change in concentration, temperature, pressure Classifying Chemical Reactions by patt ...
... Calculate equilibrium constants (given concentrations of reactants/products) Interpret equilibrium constants (large/small are products/reactants favored?) LeChatelier’s Principle -shift Left or Right in response to change in concentration, temperature, pressure Classifying Chemical Reactions by patt ...
Give formulas of these acids, bases and salts boron silicide
... 1. A solution has a pH of 4 - what does this mean? It is acidic. It is neutral. It is alkaline. 2. Which of the statements below is correct? Bases are acids that dissolve in water. Bases are alkalis that dissolve in water. Alkalis are bases that dissolve in water. 3. A liquid has a pH of 7. What doe ...
... 1. A solution has a pH of 4 - what does this mean? It is acidic. It is neutral. It is alkaline. 2. Which of the statements below is correct? Bases are acids that dissolve in water. Bases are alkalis that dissolve in water. Alkalis are bases that dissolve in water. 3. A liquid has a pH of 7. What doe ...
Acid and Bases 2
... hydroxide ions in solution. Example: HCl + NaOH NaCl + H2O **BASICALLY…acids start with H+ and bases end with OH-!!! ...
... hydroxide ions in solution. Example: HCl + NaOH NaCl + H2O **BASICALLY…acids start with H+ and bases end with OH-!!! ...
Acids and Bases
... Dissociation • In water all ionic compounds dissociate into its ionic parts • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
... Dissociation • In water all ionic compounds dissociate into its ionic parts • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
7.2 Acids and Bases
... Acids Reacts with metals and carbonates Conducts electricity Turns blue litmus paper red Tastes sour pH < 7 Neutralizes bases ...
... Acids Reacts with metals and carbonates Conducts electricity Turns blue litmus paper red Tastes sour pH < 7 Neutralizes bases ...
u11_tqs
... 13. Adding a product to an equilibrium system pushes the reaction in the direction of… reactants. Removing a product from an equilibrium system pushes the reaction in the direction of… products. Adding a reactant to an equilibrium system pushes the reaction in the direction of… products. Removing a ...
... 13. Adding a product to an equilibrium system pushes the reaction in the direction of… reactants. Removing a product from an equilibrium system pushes the reaction in the direction of… products. Adding a reactant to an equilibrium system pushes the reaction in the direction of… products. Removing a ...
Study Questions
... concentration of AgNO3 does a precipitate start to form? 9. A solution is prepared by mixing 50.0 ml of 0.0100 M lead(II) nitrate with 50.0 ml of 0.0200 M sodium bromide. Will a precipitate form? 10. Write balanced chemical equations for a) the reaction when strong acid is added to H2CO3/NaHCO3 buff ...
... concentration of AgNO3 does a precipitate start to form? 9. A solution is prepared by mixing 50.0 ml of 0.0100 M lead(II) nitrate with 50.0 ml of 0.0200 M sodium bromide. Will a precipitate form? 10. Write balanced chemical equations for a) the reaction when strong acid is added to H2CO3/NaHCO3 buff ...
Buffers and Acid/Base
... Confusion Alert! We do not use QCK tables for SA/SB calculations b/c they completely dissociate, no equilibrium so… [ACID] = [H+] Hydrolysis of Salts **Conjugates of weak acids/bases react with water to form acidic/basic solutions** ...
... Confusion Alert! We do not use QCK tables for SA/SB calculations b/c they completely dissociate, no equilibrium so… [ACID] = [H+] Hydrolysis of Salts **Conjugates of weak acids/bases react with water to form acidic/basic solutions** ...