* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download K b
Ultraviolet–visible spectroscopy wikipedia , lookup
Ionic liquid wikipedia , lookup
Ionic compound wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Sulfuric acid wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
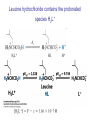
CHEMISTRY 59-320 ANALYTICAL CHEMISTRY Fall - 2010 Lecture 14 9.2 Weak acids and bases • The relationship between Ka and Kb for a conjugate acid-base pair: Ka*Kb = Kw. • Weak is conjugate to weak. The conjugate base of a weak acid is a weak base and vice verse. 9-3 Weak acid equilibria • Why is the ortho isomer 30 times more acidic than the para isomer? The product of the acid dissociation reaction forms a strong, internal hydrogen bond, that drives the reaction forward. Calculate the pH of a weak acid For any respectable weak acid, [H+] from HA will be much greater than [H+] from H2O If dissociation of HA is much greater than H2O dissociation, [A-] >>[OH-] Example: Calculation the pH of 0.0500 M o-hydroxybenzoic acid solution. The Ka = 1.0 x 10-3. Solution: plug into the above equation with F= 0.0500 and Ka = 1.0 x 10-3 The fraction of dissociation, α, is defined as the fraction of the acid in the form A− • Fraction of dissociation of a weak electrolyte increases as electrolyte is diluted. The stronger acid is more dissociated than the weaker acid at all concentrations. • Weak electrolytes (compounds that are only partially dissociated) dissociate more as they are diluted. 9-4 Weak base equilibria • Calling the formal concentration of base F (= [B] + [BH+]), • Notice that approximation in the last equation looks like a weak-acid problem, except that now x = [OH−]. 9-5 Buffers • A buffered solution resists changes in pH when acids or bases are added or when dilution occurs. • The buffer is a mixture of an acid and its conjugate base. There must be comparable amounts of the conjugate acid and base (say, within a factor of 10) to exert significant buffering. Henderson-Hasselbalch Equation • Regardless of how complex a solution may be, whenever pH = pKa, [A−] must equal [HA]. Notice that large changes in concentration Lead to rather small change in pH! • This relation is true because all equilibria must be satisfied simultaneously in any solution at equilibrium. If there are 10 different acids and bases in the solution, there is only one pH, because there can be only one concentration of H+ in a solution. Buffer properties • Buffer capacity, β, is a measure of how well a solution resists changes in pH when strong acid or base is added. • where Ca and Cb are the number of moles of strong acid and strong base per liter needed to produce a unit change in pH. • (a) Cb versus pH for a solution containing 0.100 F HA with pKa = 5.00. (b) Buffer capacity versus pH for the same system reaches a maximum when pH = pKa. • The lower curve is the derivative of the upper curve. Buffer pH Depends on Ionic Strength and Temperature • The correct Henderson-Hasselbalch equation, includes activity coefficients. Failure to include activity coefficients is the principal reason why calculated pH values do not agree with measured values. • The H2PO4-/H2PO42- buffer has a pKa of 7.20 at 0 ionic strength. At 0.1 M ionic strength, the pH of a 1:1 mole mixture of this buffer is 6.86. Molecular biology lab manuals list pKa for phosphoric acid as 6.86, which is representative for ionic strengths employed in the lab. As another example of ionic strength effects, when a 0.5 M stock solution of phosphate buffer at pH 6.6 is diluted to 0.05 M, the pH rises to 6.9—a rather significant effect. Changing ionic strength changes pH. Buffer pKa depends on temperature. Tris has an exceptionally large dependence, −0.028 pKa units per degree, near room temperature. A solution of tris with pH 8.07 at 25°C will have pH ≈ 8.7 at 4°C and pH ≈ 7.7 at 37°C. Changing temperature changes pH. • • • • Tris • A Dilute Buffer Prepared from a Moderately Strong Acid. • What will be the pH if 0.010 0 mol of HA (with pKa = 2.00) and 0.010 0 mol of A− are dissolved in water to make 1.00 L of solution? Neglecting OH- • The concentrations of HA and A− are not what we mixed. Chapter 10: Polyprotic acids and bases • Polyprotic systems are acids or bases that can donate or accept more than one proton. • A molecule that can both donate and accept a proton is said to be amphiprotic. • • • (a) How many grams of NaHCO3 (FM 84.007) must be added to 4.00 g of K2CO3 (FM 138.206) to give a pH of 10.80 in 500 mL of water? (b) What will be the pH if 100 mL of 0.100 M HCl are added to the solution in part (a)? (c) How many milliliters of 0.320 M HNO3 should be added to 4.00 g of K2CO3 to give a pH of 10.00 in 250 mL? (a) (b) (c) pH in polyprotic acid solutions The Acidic Form, H2L+ - diprotic acid Example: calculate the pH and composition of individual solutions of 0.050 0 M H2L+, 0.050 0 M HL, and 0.050 0 ML−. Leucine hydrochloride contains the protonated species H2L+ HL is an even weaker acid, because K2 = 1.80 × 10−10. Monobasic species • We can treat L− as a monobasic species, with Kb = Kb1. Great approximation! Amphiprotic character Solving for H+






















![Biochemistry 311 Problem Set: pH and Buffer 1. Calculate the [H+] of](http://s1.studyres.com/store/data/016276514_1-cc9bfff072c2adb68721959b3f97d8e4-150x150.png)








