CH 151 Companion
... f. Do not put pipets directly in any reagent bottle. This might result in contamination of the remaining liquid in the bottle. Never mouth pipet any liquid in the lab. g. Keep the lids and caps on the chemical bottles. Put the lids back on as soon as you are finished dispensing the material. Many ch ...
... f. Do not put pipets directly in any reagent bottle. This might result in contamination of the remaining liquid in the bottle. Never mouth pipet any liquid in the lab. g. Keep the lids and caps on the chemical bottles. Put the lids back on as soon as you are finished dispensing the material. Many ch ...
CHAPTER 16 ACID-BASE EQUILIBRIA AND SOLUBILITY
... Could you have predicted whether the pH should have increased or decreased after the addition of the sodium acetate to the pure 0.40 M acetic acid in part (a)? An alternate way to work part (b) of this problem is to use the Henderson-Hasselbalch equation. pH = pKa + log ...
... Could you have predicted whether the pH should have increased or decreased after the addition of the sodium acetate to the pure 0.40 M acetic acid in part (a)? An alternate way to work part (b) of this problem is to use the Henderson-Hasselbalch equation. pH = pKa + log ...
Midterm Practice Exam Key
... 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which the aqueous (dissolved) ions ...
... 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which the aqueous (dissolved) ions ...
Equilibrium
... Both the forward and reverse reactions continue, but because their rates are equal, no net change occurs in their concentrations. Even though the rates are equal at equilibrium, the concentrations of the components on both side of the equation are not necessarily the same. The relative concentration ...
... Both the forward and reverse reactions continue, but because their rates are equal, no net change occurs in their concentrations. Even though the rates are equal at equilibrium, the concentrations of the components on both side of the equation are not necessarily the same. The relative concentration ...
ISOTONICITY
... same osmotic pressure as that of 1 g of the chemical. The sodium chloride equivalents are symbolized by the letter E. The quantities of two substances that are isotonic equivalents are proportional to the molecular weight of each multiplied by the i value of the other. Thus, if the molecular w ...
... same osmotic pressure as that of 1 g of the chemical. The sodium chloride equivalents are symbolized by the letter E. The quantities of two substances that are isotonic equivalents are proportional to the molecular weight of each multiplied by the i value of the other. Thus, if the molecular w ...
ap chemistry 2005/2006
... thermochemical reactions, identification of endothermic or exothermic, identification as physical or chemical change. Lab: Determining the Specific Heat of an Unknown Metal – the specific heat capacity of a nail will be experimentally determined by measuring the temperature change of water and of th ...
... thermochemical reactions, identification of endothermic or exothermic, identification as physical or chemical change. Lab: Determining the Specific Heat of an Unknown Metal – the specific heat capacity of a nail will be experimentally determined by measuring the temperature change of water and of th ...
GROUP 13 ELEMENTS -THE BORON FAMILY -
... important because it forms gallium arsenide (GaAs), which can convert light directly into electricity. Also due to thermite reaction, aluminum can extract oxygen from water and hydrogen is released. However, as mentioned above, aluminum forms a protective coat in the presence of water. By combining ...
... important because it forms gallium arsenide (GaAs), which can convert light directly into electricity. Also due to thermite reaction, aluminum can extract oxygen from water and hydrogen is released. However, as mentioned above, aluminum forms a protective coat in the presence of water. By combining ...
Solubility
... – Ppt of CaCO3 from groundwater is responsible for cave formation. Let’s look at the factors that affect solubility! ...
... – Ppt of CaCO3 from groundwater is responsible for cave formation. Let’s look at the factors that affect solubility! ...
[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION
... 1)Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2)Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3)Balanced Chemical e ...
... 1)Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2)Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3)Balanced Chemical e ...
Appendix
... at the end point, the purity of the KHP, the molar mass for KHP, and the titration’s repeatability. Having established these, we can combine them to arrive at the final uncertainty. Uncertainty in the Mass of KHP. After drying the KHP, we store it in a sealed container to prevent it from readsorbing ...
... at the end point, the purity of the KHP, the molar mass for KHP, and the titration’s repeatability. Having established these, we can combine them to arrive at the final uncertainty. Uncertainty in the Mass of KHP. After drying the KHP, we store it in a sealed container to prevent it from readsorbing ...
Spontaniety Worked Examples
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
chemical reactions and stoichiometry chemical reactions and
... The equation is not balanced, because there are too many carbon and hydrogen atoms on the left and too many oxygen atoms on the right. We need to change the numbers of molecules by changing stoichiometric coefficients until the numbers of atoms of each element are equal. It is easiest to balance a c ...
... The equation is not balanced, because there are too many carbon and hydrogen atoms on the left and too many oxygen atoms on the right. We need to change the numbers of molecules by changing stoichiometric coefficients until the numbers of atoms of each element are equal. It is easiest to balance a c ...
Inorganic Chemistry‑II
... Course Chem-201AH (class assessment) includes tutorial, terminal, home assignment and /or class examinations on theoretical courses by the relevant course teacher(s) and attendance* of the students in the classes during the academic year. Class assessment comprises (a) 80% marks in tutorial, termina ...
... Course Chem-201AH (class assessment) includes tutorial, terminal, home assignment and /or class examinations on theoretical courses by the relevant course teacher(s) and attendance* of the students in the classes during the academic year. Class assessment comprises (a) 80% marks in tutorial, termina ...
CHEMISTRY
... Explain why it is a liquid at room temperature. iv. Oils, such as vegetable oils, can be modified chemically to make margarine, that is they can be turned into fats (solids). Name the chemical reagent necessary to carry out this transformation. ...
... Explain why it is a liquid at room temperature. iv. Oils, such as vegetable oils, can be modified chemically to make margarine, that is they can be turned into fats (solids). Name the chemical reagent necessary to carry out this transformation. ...
Molarity Practice Worksheet
... The equation for molarity states that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. In the first equation, the molarity will clearly be equal to 1.0 M, because there are 1.0 moles of NaCl and a solution volume of 1.0 L. In the secon ...
... The equation for molarity states that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. In the first equation, the molarity will clearly be equal to 1.0 M, because there are 1.0 moles of NaCl and a solution volume of 1.0 L. In the secon ...
Dangerous Goods - `OnGuard®` Safety Training
... rain. Appropriate spillage clean-up and personal protective equipment should be provided and kept in good order. The type of clean-up and personal protective equipment required, willdepend on the quantities of DG, their types and handling methods. The PPE should usually include corrosive resistant g ...
... rain. Appropriate spillage clean-up and personal protective equipment should be provided and kept in good order. The type of clean-up and personal protective equipment required, willdepend on the quantities of DG, their types and handling methods. The PPE should usually include corrosive resistant g ...
Reactions and Solutions - Louisiana Tech University
... The majority of chemical reactions, and virtually all important organic and biochemical processes, occur in solution. A solution is composed of one or more solutes dissolved in a solvent. In aqueous solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles ...
... The majority of chemical reactions, and virtually all important organic and biochemical processes, occur in solution. A solution is composed of one or more solutes dissolved in a solvent. In aqueous solutions, the solvent is water. Liquid solutions are clear and transparent with no visible particles ...
chap15pptlecture_chapte.ppt [Read-Only]
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
Detection of Organic Pollutants with a Pulsed Ion Mobility
... for high concentrations, there is only one peak at the same position, which is the monomer peak of formic acid. At high concentrations the reactant ions are completely gone so there is no RIP peak, at low concentrations there are still reactant ions left after the ionization of the analyte molecules ...
... for high concentrations, there is only one peak at the same position, which is the monomer peak of formic acid. At high concentrations the reactant ions are completely gone so there is no RIP peak, at low concentrations there are still reactant ions left after the ionization of the analyte molecules ...
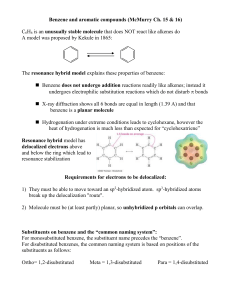
Ex: -F, -Cl, -Br
... Compounds which fit the following criteria can be considered aromatic: 1) Cyclic and planar 2) Continuous overlap of unhybridized p-orbitals forming a delocalized -cloud of e3) The number of -electrons must be equal to 4n + 2 (where n = 0, 1, 2…) Huckel’s Rule (4n + 2 rule) relates to the way el ...
... Compounds which fit the following criteria can be considered aromatic: 1) Cyclic and planar 2) Continuous overlap of unhybridized p-orbitals forming a delocalized -cloud of e3) The number of -electrons must be equal to 4n + 2 (where n = 0, 1, 2…) Huckel’s Rule (4n + 2 rule) relates to the way el ...









![[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION](http://s1.studyres.com/store/data/008930072_1-5a35e1ae8e3204ea88999f1418a93013-300x300.png)










![chap15pptlecture_chapte.ppt [Read-Only]](http://s1.studyres.com/store/data/015369082_1-00cbf06a2d468a4ae1c963f5ca674e31-300x300.png)