Chapter 17 lecture notes on Chemical Equilibria
... A few points to make about Kc: 1. Temperature dependence. The value is obtained for a specific temperature. This is not surprising since K is a thermodynamic constant and all thermodynamic constants are strongly dependent upon T. Once again, a standard temperature has to be selected for recording v ...
... A few points to make about Kc: 1. Temperature dependence. The value is obtained for a specific temperature. This is not surprising since K is a thermodynamic constant and all thermodynamic constants are strongly dependent upon T. Once again, a standard temperature has to be selected for recording v ...
Unit 4 - Chemical Equilibrium
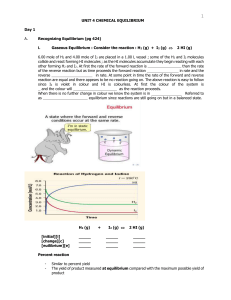
... N2 ( g ) + 3 H2 (g) 2 NH3 ( g ) increase conc. of N2 -----> stress too much to relieve stress equilibrium shifts to the ; thus conc. of H2 will and conc. of NH3 will ____________ Consider the equ. conc to be N2 = 1.0 moL/L; H2 = 3.0 moL/L and NH3 = 2.0 moL/L If an additional 0.5 moL/L of N2 is add ...
... N2 ( g ) + 3 H2 (g) 2 NH3 ( g ) increase conc. of N2 -----> stress too much to relieve stress equilibrium shifts to the ; thus conc. of H2 will and conc. of NH3 will ____________ Consider the equ. conc to be N2 = 1.0 moL/L; H2 = 3.0 moL/L and NH3 = 2.0 moL/L If an additional 0.5 moL/L of N2 is add ...
Chapter 1: Aqueous Processing Systems
... One advantage of the established situation is that the student is exposed to aqueous processing in context, i.e., there can be immediate connection with the student's chosen field. Unfortunately, however, this approach to technical education is not without some disadvantages. First of all, many aqu ...
... One advantage of the established situation is that the student is exposed to aqueous processing in context, i.e., there can be immediate connection with the student's chosen field. Unfortunately, however, this approach to technical education is not without some disadvantages. First of all, many aqu ...
CHE 1402 Lab Manual
... where a and b are constants characteristic of a given gas. The term nb is a correction for the finite n2 volume of the molecules. The term a 2 is a correction to the pressure which takes into account the V intermolecular attractions. In this experiment you will determine the numerical value of the g ...
... where a and b are constants characteristic of a given gas. The term nb is a correction for the finite n2 volume of the molecules. The term a 2 is a correction to the pressure which takes into account the V intermolecular attractions. In this experiment you will determine the numerical value of the g ...
What is equilibrium?
... is different from the moles of gaseous products. • If the number of moles is the same on both sides of the balanced equation, changes in pressure and volume have no effect on the equilibrium. ...
... is different from the moles of gaseous products. • If the number of moles is the same on both sides of the balanced equation, changes in pressure and volume have no effect on the equilibrium. ...
Document
... An exothermic reaction is exothermic, no matter what the temperature is. An endothermic reaction is endothermic at all temperatures. You should think of heat as ________________for endothermic reactions and as ________________ for exothermic reactions. B. Le Châtelier's principle - add hea ...
... An exothermic reaction is exothermic, no matter what the temperature is. An endothermic reaction is endothermic at all temperatures. You should think of heat as ________________for endothermic reactions and as ________________ for exothermic reactions. B. Le Châtelier's principle - add hea ...
- Kendriya Vidyalaya Damoh
... (i) Reaction with Metals – Certain metals such as Zinc, Aluminiumand Tin react with alkali solutions on heating and hydrogen gas is evolved (ii) Reaction with acids – Bases react with acids to form salt and water. Indicators - Indicators are substances which indicate the acidic or basic nature of th ...
... (i) Reaction with Metals – Certain metals such as Zinc, Aluminiumand Tin react with alkali solutions on heating and hydrogen gas is evolved (ii) Reaction with acids – Bases react with acids to form salt and water. Indicators - Indicators are substances which indicate the acidic or basic nature of th ...
- Kendriya Vidyalaya No.1, Satna
... (i) Reaction with Metals – Certain metals such as Zinc, Aluminiumand Tin react with alkali solutions on heating and hydrogen gas is evolved (ii) Reaction with acids – Bases react with acids to form salt and water. Indicators - Indicators are substances which indicate the acidic or basic nature of th ...
... (i) Reaction with Metals – Certain metals such as Zinc, Aluminiumand Tin react with alkali solutions on heating and hydrogen gas is evolved (ii) Reaction with acids – Bases react with acids to form salt and water. Indicators - Indicators are substances which indicate the acidic or basic nature of th ...
Practice Exam I FR Answers and Explanations
... (a) Predict sign of Eº and explain. The sign of Eº must be positive. The prompt gives a K value of 1.5 × 1011 which means that the products are favored at equilibrium. Since the reaction proceeds as written, the voltage should be positive. (b) Identify reducing agent. Cd changes oxidation states fro ...
... (a) Predict sign of Eº and explain. The sign of Eº must be positive. The prompt gives a K value of 1.5 × 1011 which means that the products are favored at equilibrium. Since the reaction proceeds as written, the voltage should be positive. (b) Identify reducing agent. Cd changes oxidation states fro ...
Chapter 4
... the balance of all atoms and charges. Multiply the balanced half-reactions by appropriate coefficients to make the number of electrons cancel. In this case, multiply the reduction by 3 and the oxidation by 4 for a total of 24 electrons on each side. 3 x [8 OH- + N2H4 → 2 NO + 8 e- + 6 H2O] i.e., 24 ...
... the balance of all atoms and charges. Multiply the balanced half-reactions by appropriate coefficients to make the number of electrons cancel. In this case, multiply the reduction by 3 and the oxidation by 4 for a total of 24 electrons on each side. 3 x [8 OH- + N2H4 → 2 NO + 8 e- + 6 H2O] i.e., 24 ...
Document
... thereby selects for pathogenic Salmonella (bile-resistant growth) present in fecal specimens. • Salmonella species as non-lactose and non-sucrose fermenters that produce H2S form colorless colonies with black centers. • Shigella species (non-lactose and non-sucrose fermenters, no H2S production) for ...
... thereby selects for pathogenic Salmonella (bile-resistant growth) present in fecal specimens. • Salmonella species as non-lactose and non-sucrose fermenters that produce H2S form colorless colonies with black centers. • Shigella species (non-lactose and non-sucrose fermenters, no H2S production) for ...
Unit 6: Solution Chemistry Content Outline: Basic Solution Chemistry
... The precipitate occurs when the inter-molecular attractions between the ions is greater than the inter-molecular attractions between the ions and surrounding water molecules. III. Ionization (The “making” of ions.) A. Ions are “formed” from a molecular compound (covalent bonds) solute by the action ...
... The precipitate occurs when the inter-molecular attractions between the ions is greater than the inter-molecular attractions between the ions and surrounding water molecules. III. Ionization (The “making” of ions.) A. Ions are “formed” from a molecular compound (covalent bonds) solute by the action ...
Chemistry booklet
... The mole concept gives us a means of managing fixed numbers of atoms / ions / molecules in ...
... The mole concept gives us a means of managing fixed numbers of atoms / ions / molecules in ...
Intro to Titrimetry
... 1. A Fajans titration of a 0.7908 g sample required 45.32 mL of 0.1046 M AgNO3. What is the %Cl of the sample? 2. The bismuth in 0.7405 g of an alloy was precipitated as BiOCl and separated from the solution by filtration. The washed precipitate was dissolved in nitric acid to convert all chlorine t ...
... 1. A Fajans titration of a 0.7908 g sample required 45.32 mL of 0.1046 M AgNO3. What is the %Cl of the sample? 2. The bismuth in 0.7405 g of an alloy was precipitated as BiOCl and separated from the solution by filtration. The washed precipitate was dissolved in nitric acid to convert all chlorine t ...