Final Review 3-8 Answers_2
... c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass of solute in a specific volume of solution is called a) titration b) crystallization c) filtration d) distillation 5. In the analysis section of an investigation report, a) q ...
... c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass of solute in a specific volume of solution is called a) titration b) crystallization c) filtration d) distillation 5. In the analysis section of an investigation report, a) q ...
Organic Chemistry/Fourth Edition: e-Text
... Conversion of Acyl Chlorides to Other Carboxylic Acid Derivatives General equation and specific example ...
... Conversion of Acyl Chlorides to Other Carboxylic Acid Derivatives General equation and specific example ...
EDEXCEL A LeveL - Hodder Education
... The key to working with chemical amounts in moles is to know the relative masses of different atoms. The accurate method for determining relative atomic masses involves the use of a mass spectrometer (Section 1.3). One mole of an element has a mass that is equal to its relative atomic mass in grams. ...
... The key to working with chemical amounts in moles is to know the relative masses of different atoms. The accurate method for determining relative atomic masses involves the use of a mass spectrometer (Section 1.3). One mole of an element has a mass that is equal to its relative atomic mass in grams. ...
Equilibrium notes (complete)
... • http://www.mhhe.com/physsci/chemistry/animations/chang_ 7e_esp/kim2s2_5.swf reversible-reactions.jar Static equilibrium (Physics) • No changes occur at all Assign 3-5 ...
... • http://www.mhhe.com/physsci/chemistry/animations/chang_ 7e_esp/kim2s2_5.swf reversible-reactions.jar Static equilibrium (Physics) • No changes occur at all Assign 3-5 ...
Kinetics of Oxidation of Benzyl Alcohol with Dilute Nitric Acid
... that the gas phase did not contain NO, NO2, N2O4, or N2O5. Thus, it may be concluded that the NOx that is liberated is in the form of N2O. The liberated N2O in the product gas mixture can be used as a clean and selective oxidant, e.g., for the selective oxidative coupling of methane to ethane (Sugiy ...
... that the gas phase did not contain NO, NO2, N2O4, or N2O5. Thus, it may be concluded that the NOx that is liberated is in the form of N2O. The liberated N2O in the product gas mixture can be used as a clean and selective oxidant, e.g., for the selective oxidative coupling of methane to ethane (Sugiy ...
Oxidation-reduction reaction of chromium (VI) and iron (III) with
... increase in [H+] as shown in tables 3a and 3babove. Plots of logkobs vs. log[H+] in this acid concentration range are linear with a slope of 1 implying that the reactions are first order with respect to hydrogen ionsfor dichromate ion and iron (III) ion. The increase in rate constant with increasing ...
... increase in [H+] as shown in tables 3a and 3babove. Plots of logkobs vs. log[H+] in this acid concentration range are linear with a slope of 1 implying that the reactions are first order with respect to hydrogen ionsfor dichromate ion and iron (III) ion. The increase in rate constant with increasing ...
The Major Classes of Chemical Reactions
... how water acts as a solvent. The role a solvent plays in a reaction depends on its chemical nature. Some solvents play a passive role. They disperse the substances into individual molecules but do not interact with them in other ways. Water plays a much more active role. It interacts strongly with t ...
... how water acts as a solvent. The role a solvent plays in a reaction depends on its chemical nature. Some solvents play a passive role. They disperse the substances into individual molecules but do not interact with them in other ways. Water plays a much more active role. It interacts strongly with t ...
Chemistry IGCSE
... enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut. In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regular ...
... enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut. In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regular ...
AP Chemistry
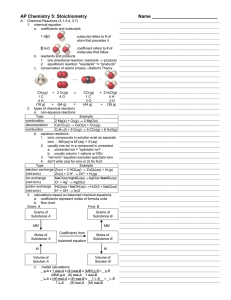
... each element on each side of the equation. Equations are balanced by placing coefficients in front of the chemical formulas for the reactants and products of a reaction, not by changing the subscripts in chemical formulas. Among the reaction types described in this unit are (1) combination reactions ...
... each element on each side of the equation. Equations are balanced by placing coefficients in front of the chemical formulas for the reactants and products of a reaction, not by changing the subscripts in chemical formulas. Among the reaction types described in this unit are (1) combination reactions ...
sch103manual - university of nairobi staff profiles
... The above equation is the mathematical expression of Avogadro’s Law, which states that at constant pressure and temperature, the volume of a gas is directly proportional to the number of moles of the gas present. One mole of any gas contains the same number of molecules ( Avogadro’s number = 6.022 ...
... The above equation is the mathematical expression of Avogadro’s Law, which states that at constant pressure and temperature, the volume of a gas is directly proportional to the number of moles of the gas present. One mole of any gas contains the same number of molecules ( Avogadro’s number = 6.022 ...
Chapter 17 - Cengage Learning
... reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction to occur, and this is termed the activation energy. As the temperature increases, the molecules absorb mor ...
... reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction to occur, and this is termed the activation energy. As the temperature increases, the molecules absorb mor ...
EXPERIMENT 10 Volumetric Analysis I Standardization of NaOH
... Introduction Titration is a common method of quantitative analysis used to determine the concentration of an unknown substance in a solution. The method is easy to use if the quantitative relationship between two reacting solutions is known. It is particularly well-suited to acid-base and oxidation- ...
... Introduction Titration is a common method of quantitative analysis used to determine the concentration of an unknown substance in a solution. The method is easy to use if the quantitative relationship between two reacting solutions is known. It is particularly well-suited to acid-base and oxidation- ...
Practice Test Material - Directorate of Education
... Calculate the pH of 0.10M ammonia solution. Calculate the pH after 50.0 ml of this solution is treated with 25.0 ml of 0.10M HCl. The dissociation constant of ammonia (Kb) is 1.77×10–5. Hint – In the final condition, basic buffer is formed due to the presence of NH4Cl and NH4OH in the same solution. ...
... Calculate the pH of 0.10M ammonia solution. Calculate the pH after 50.0 ml of this solution is treated with 25.0 ml of 0.10M HCl. The dissociation constant of ammonia (Kb) is 1.77×10–5. Hint – In the final condition, basic buffer is formed due to the presence of NH4Cl and NH4OH in the same solution. ...
Sugar Amino Acids - The Krasavin research group
... Most research in the field of β-SAAs has concentrated on the furanosidic scaffold. In the group of β-SAAs encompassing both furanosidic and pyranosidic structures, two major subgroups can be ascribed as a function of the position of the carboxylic and amino functions, one group embracing all the com ...
... Most research in the field of β-SAAs has concentrated on the furanosidic scaffold. In the group of β-SAAs encompassing both furanosidic and pyranosidic structures, two major subgroups can be ascribed as a function of the position of the carboxylic and amino functions, one group embracing all the com ...
Chapter 4
... This equation says that all sodium chloride that enters the solution ends up as Na1 and Cl2 ions; there are no undissociated NaCl units in solution. Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI ...
... This equation says that all sodium chloride that enters the solution ends up as Na1 and Cl2 ions; there are no undissociated NaCl units in solution. Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI ...
DUE: Tuesday, Jan. 20, 2015 Solutions Take Home Test
... ____ 11. number of moles of solute dissolved in 1 L of solution ____ 12. a colligative property related to the fact that ice will form at higher temperatures in the Great Lakes than in the ocean ____ 13. a colligative property related to a decrease in the vapor pressure of a solution ...
... ____ 11. number of moles of solute dissolved in 1 L of solution ____ 12. a colligative property related to the fact that ice will form at higher temperatures in the Great Lakes than in the ocean ____ 13. a colligative property related to a decrease in the vapor pressure of a solution ...
Unit F325 - Equilibria, energetics and elements - High band
... Lattice enthalpies are negative values and use of the words ‘more’ and ‘less’ should not be used unless qualified for comparing negative numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘le ...
... Lattice enthalpies are negative values and use of the words ‘more’ and ‘less’ should not be used unless qualified for comparing negative numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘le ...
CHEMISTRY OF p-ELEMENTS - Львівський національний
... Hans Christian Oersted, a Danish chemist, was the first who isolated aluminum in 1825, using a chemical process involving potassium amalgam. Between 1827 and 1845, Friedrich Wohler, a German chemist, improved Oersted's process by using metallic potassium. He was the first to measure the specific gr ...
... Hans Christian Oersted, a Danish chemist, was the first who isolated aluminum in 1825, using a chemical process involving potassium amalgam. Between 1827 and 1845, Friedrich Wohler, a German chemist, improved Oersted's process by using metallic potassium. He was the first to measure the specific gr ...
articles - Geoscience Research Institute
... containing many of the biochemical pathways fundamental to life began to flourish. Direct experimental evidence seeming to validate the Oparin-Haldane hypothesis was first produced in 1953 by S. L. Miller.6 This led to many other laboratory investigations of the prebiotic precursors that are thought ...
... containing many of the biochemical pathways fundamental to life began to flourish. Direct experimental evidence seeming to validate the Oparin-Haldane hypothesis was first produced in 1953 by S. L. Miller.6 This led to many other laboratory investigations of the prebiotic precursors that are thought ...
COMPLEX IONS AND AMPHOTERISM
... An amphoteric substance is one that can behave as a Lewis acid and a Brønsted base. The best examples are found with metal hydroxides such as aluminum hydroxide [Al(OH)3] and zinc hydroxide [Zn(OH)2]. Insoluble aluminum hydroxide can be formed by the addition of hydroxide ion, OH-, to a soluble salt ...
... An amphoteric substance is one that can behave as a Lewis acid and a Brønsted base. The best examples are found with metal hydroxides such as aluminum hydroxide [Al(OH)3] and zinc hydroxide [Zn(OH)2]. Insoluble aluminum hydroxide can be formed by the addition of hydroxide ion, OH-, to a soluble salt ...
The Potential Contribution of Organic Salts to New
... compositions, as such salts may be important contributors to new particle growth. ...
... compositions, as such salts may be important contributors to new particle growth. ...
QualGroupD
... cations in your unknown. Testing known solutions to determine the color of the complex ions formed is recommended. Magnesium hydroxide will precipitate in aqueous ammonia, however the precipitate can be prevented from forming if the solution contains a significant concentration of NH4+. This can be ...
... cations in your unknown. Testing known solutions to determine the color of the complex ions formed is recommended. Magnesium hydroxide will precipitate in aqueous ammonia, however the precipitate can be prevented from forming if the solution contains a significant concentration of NH4+. This can be ...
AP Chemistry
... 0.1 M AgNO3 and 15 drops of 0.1 M K2CrO4. Stopper the tube and shake periodically for 10 minutes. Centrifuge for 3 minutes (be sure a test tube with similar amount of liquid is in the slot opposite your tube). Pour off the liquid, called the supernatant, while leaving the precipitate in the test tub ...
... 0.1 M AgNO3 and 15 drops of 0.1 M K2CrO4. Stopper the tube and shake periodically for 10 minutes. Centrifuge for 3 minutes (be sure a test tube with similar amount of liquid is in the slot opposite your tube). Pour off the liquid, called the supernatant, while leaving the precipitate in the test tub ...