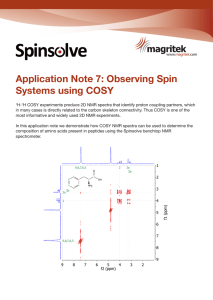
Application Note 7: Observing Spin Systems using COSY
... systems are shown in different colours, and coupling is where the lines intersect on a peak off the diagonal (crosspeak). The protons in line with this cross-peak couple to each other. Note that the methylene protons occur at different chemical shifts despite being attached to the same carbon atom, ...
... systems are shown in different colours, and coupling is where the lines intersect on a peak off the diagonal (crosspeak). The protons in line with this cross-peak couple to each other. Note that the methylene protons occur at different chemical shifts despite being attached to the same carbon atom, ...
aq - Byron High School
... 3b). Because the H2S molecule is neutral, the sum of the oxidation numbers must equal zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) ...
... 3b). Because the H2S molecule is neutral, the sum of the oxidation numbers must equal zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) ...
Lab # 18
... b. What number is represented when there is no coefficient written? 7. What is a subscript? 8. What is a diatomic molecule? 9. What phase are most diatomic molecules found in? 10. What is a chemical equation? 11. How can you tell whether an equation is balanced? 12. What can an equation tell us abou ...
... b. What number is represented when there is no coefficient written? 7. What is a subscript? 8. What is a diatomic molecule? 9. What phase are most diatomic molecules found in? 10. What is a chemical equation? 11. How can you tell whether an equation is balanced? 12. What can an equation tell us abou ...
A matter of Equilibrium
... It is more convenient to introduce a logarithmic scale, the pH scale, where: ...
... It is more convenient to introduce a logarithmic scale, the pH scale, where: ...
Chapter 4 Lecture Notes in PowerPoint
... Representing Aqueous Reactions • An equation showing the complete neutral formulas for each compound in the aqueous reaction as if they existed as molecules is called a molecular equation. 2 KOH(aq) + Mg(NO3)2(aq) 2 KNO3(aq) + Mg(OH)2(s) ...
... Representing Aqueous Reactions • An equation showing the complete neutral formulas for each compound in the aqueous reaction as if they existed as molecules is called a molecular equation. 2 KOH(aq) + Mg(NO3)2(aq) 2 KNO3(aq) + Mg(OH)2(s) ...
Aquo complexes of simple Cu+, Ag+ and Aut ions and the acidities
... since according to our PM3 calcu lations the con'esponding monohydroxo species are not stable in the gas phase. From Fig. 2, it is apparent that the aqueous solution of a simple metal salt becomes more acidic as the gas phase thermochemical feasibility of the loss of a proton from its aquo compl ex ...
... since according to our PM3 calcu lations the con'esponding monohydroxo species are not stable in the gas phase. From Fig. 2, it is apparent that the aqueous solution of a simple metal salt becomes more acidic as the gas phase thermochemical feasibility of the loss of a proton from its aquo compl ex ...
85 Q.2 Pure water has a low electricity conductivity because A. it
... Which of the following statements concerning 25 cm3 of 1M hydrochloric acid and 25 cm3 of 1M ethanoic acid is / are correct? (1) They give the same colour change when the same quantity to universal indicator is added. (2) They react with marble chips at the same rate when the initial temperatures ar ...
... Which of the following statements concerning 25 cm3 of 1M hydrochloric acid and 25 cm3 of 1M ethanoic acid is / are correct? (1) They give the same colour change when the same quantity to universal indicator is added. (2) They react with marble chips at the same rate when the initial temperatures ar ...
Keq = [A] [B] [C] [D]
... Does this make sense? Keq is very small, so the reaction will not proceed very far to the right, so the concentrations of the products will be small. Yes, this answer does make sense. Example #3: How many moles of HI are present at equilibrium when 2.0 moles of H2 is mixed with 1.0 mole of I2 in a 0 ...
... Does this make sense? Keq is very small, so the reaction will not proceed very far to the right, so the concentrations of the products will be small. Yes, this answer does make sense. Example #3: How many moles of HI are present at equilibrium when 2.0 moles of H2 is mixed with 1.0 mole of I2 in a 0 ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
Abstract
... data where the El-mass showing prominent molecular ion peak and the fragmentation pattern might represent the base peak, loss of CN, NH-NH2CH3 from the molecular ion was observed. Furthermore 2-(3-cyanquinoline-2-ylthio) acetohydrazied (VI) was allowed to condense with different aromatic aldehydes w ...
... data where the El-mass showing prominent molecular ion peak and the fragmentation pattern might represent the base peak, loss of CN, NH-NH2CH3 from the molecular ion was observed. Furthermore 2-(3-cyanquinoline-2-ylthio) acetohydrazied (VI) was allowed to condense with different aromatic aldehydes w ...
Dr. Spencer`s PPT
... Net Ionic Equations greatly simplify equations and points out similarities and differences between reactions more easily. (i.e., EVERY neutralization reaction appears the same); 2OH-(aq) + 2H+(aq) ...
... Net Ionic Equations greatly simplify equations and points out similarities and differences between reactions more easily. (i.e., EVERY neutralization reaction appears the same); 2OH-(aq) + 2H+(aq) ...
hong kong diploma of secondary education examination
... Compound Q reacts with dilute sulphuric acid to give a colourless gas R and an aqueous solution S. When the solution S is further treated with sodium hydroxide solution, it does not give a precipitate. What could this compound be? ...
... Compound Q reacts with dilute sulphuric acid to give a colourless gas R and an aqueous solution S. When the solution S is further treated with sodium hydroxide solution, it does not give a precipitate. What could this compound be? ...
Example 4
... Definitions: Standardise: means to find the concentration of a solution using titration A Titration: is a laboratory procedure where a measured volume of one solution is added to a known volume of another solution until the reaction is complete. ...
... Definitions: Standardise: means to find the concentration of a solution using titration A Titration: is a laboratory procedure where a measured volume of one solution is added to a known volume of another solution until the reaction is complete. ...
Page 1 of 9 Chem 103 Practice Problems: Below is a key for both
... Solution: Similar to the problem above. So, vrx = (1/2)(3.0x10-2)=1.5x10-2mol/min ii) the rate at which ammonium nitrate is used up. Solution: Since vrxn = (1/2)(dNH4NO3/dt) we can write: dNH4NO3/dt = 2 vrxn = 2(1.5x10-2mol/min) = 3.0x10-2mol/min iii) the rate at which oxygen is formed. Since vrxn = ...
... Solution: Similar to the problem above. So, vrx = (1/2)(3.0x10-2)=1.5x10-2mol/min ii) the rate at which ammonium nitrate is used up. Solution: Since vrxn = (1/2)(dNH4NO3/dt) we can write: dNH4NO3/dt = 2 vrxn = 2(1.5x10-2mol/min) = 3.0x10-2mol/min iii) the rate at which oxygen is formed. Since vrxn = ...














![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)