CHEMISTRY SEC 06 SYLLABUS
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
CHEMISTRY SEC 06 SYLLABUS
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
Ch 4 Student
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
CHEMISTRY SEC 06 SYLLABUS
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
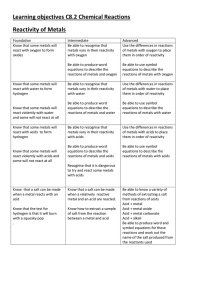
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... Be able to interpret where an unknown metal would be placed in the reactivity series based on its chemical reactions ...
... Be able to interpret where an unknown metal would be placed in the reactivity series based on its chemical reactions ...
General Concepts of Chemical Equilibrium
... Systematic Approach to Equilibrium Calculations This Type of approach to equilibrium calculations uses what is called mass balance and charge balance concepts. These will be discussed below shortly. By mass balance we mean that when we dissolve an amount of dissociating substance, the analytical co ...
... Systematic Approach to Equilibrium Calculations This Type of approach to equilibrium calculations uses what is called mass balance and charge balance concepts. These will be discussed below shortly. By mass balance we mean that when we dissolve an amount of dissociating substance, the analytical co ...
- Catalyst
... The Solubility of Covalent Compounds in Water Polar covalent compounds are very soluble in water. They often have OH groups that can form “hydrogen bonds” with water. Examples are table sugar (C12H22O11), ethanol (C2H5OH), ethylene glycol (C2H6O2) in antifreeze, and methanol (CH3OH). These also are ...
... The Solubility of Covalent Compounds in Water Polar covalent compounds are very soluble in water. They often have OH groups that can form “hydrogen bonds” with water. Examples are table sugar (C12H22O11), ethanol (C2H5OH), ethylene glycol (C2H6O2) in antifreeze, and methanol (CH3OH). These also are ...
Preparation and Properties of Hydrogen
... hydrogen will float. Because of the hydrogen molecule's small size, it will diffuse through many substances. Hydrogen gas is extremely flammable and will react with oxygen to form water with a release of a great deal of heat. The Hindenburg Zeppelin was destroyed in 1937 because of this reaction. He ...
... hydrogen will float. Because of the hydrogen molecule's small size, it will diffuse through many substances. Hydrogen gas is extremely flammable and will react with oxygen to form water with a release of a great deal of heat. The Hindenburg Zeppelin was destroyed in 1937 because of this reaction. He ...
aq - HCC Learning Web
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
... • Aqueous solutions of lead(II) nitrate and potassium iodide produce a yellow precipitate of lead(II) iodide and an aqueous solution of potassium nitrate Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) • Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calciu ...
chapter 13 - Humble ISD
... A BIT HARDER 3 NaOH (aq) + H3PO4 (aq) Na3PO4 (aq) + 3 H2O (l) Na3PO4 = 0.200 M The Keq is 130 What is the concentration of H3PO4 and NaOH? We have two unknowns so we must have two equations: First Equation is: Keq = [0.200] ...
... A BIT HARDER 3 NaOH (aq) + H3PO4 (aq) Na3PO4 (aq) + 3 H2O (l) Na3PO4 = 0.200 M The Keq is 130 What is the concentration of H3PO4 and NaOH? We have two unknowns so we must have two equations: First Equation is: Keq = [0.200] ...
Bk2P06EE
... The positive value indicates that the reaction is feasible but it gives no information about the rate. Nevertheless, the activation energy for the reaction in (a)(ii) is likely to be small since it involves simple electron transfer without involving breaking of covalent bonds. Therefore, the reactio ...
... The positive value indicates that the reaction is feasible but it gives no information about the rate. Nevertheless, the activation energy for the reaction in (a)(ii) is likely to be small since it involves simple electron transfer without involving breaking of covalent bonds. Therefore, the reactio ...
Solutions
... make conversions before you solve for and answer. For example if you are given the mass of the solute you will need to convert to moles using the mole calculation equation on Table T. You may also need to convert the volume of solution given if it is not in liters. The units for molarity are mol./L ...
... make conversions before you solve for and answer. For example if you are given the mass of the solute you will need to convert to moles using the mole calculation equation on Table T. You may also need to convert the volume of solution given if it is not in liters. The units for molarity are mol./L ...
General Equilibrium FR worksheet
... This decomposition is endothermic. A sample of 3.509 grams of SO2Cl2 is placed in an evacuated 1.00 liter bulb and the temperature is raised to 375 K. (a) What would be the pressure in atmospheres in the bulb if no dissociation of the SO2Cl2(g) occurred? (b) When the system has come to equilibrium a ...
... This decomposition is endothermic. A sample of 3.509 grams of SO2Cl2 is placed in an evacuated 1.00 liter bulb and the temperature is raised to 375 K. (a) What would be the pressure in atmospheres in the bulb if no dissociation of the SO2Cl2(g) occurred? (b) When the system has come to equilibrium a ...
Chemical Equilibrium
... The equilibrium constant of a reaction that has been multiplied by a number is the equilibrium constant raised to a power equal to that number The equilibrium constant for a net reaction of two or more steps is the product of the constants of the individual steps ...
... The equilibrium constant of a reaction that has been multiplied by a number is the equilibrium constant raised to a power equal to that number The equilibrium constant for a net reaction of two or more steps is the product of the constants of the individual steps ...
Descriptive Chemistry of Elements p
... Buckminster fullerene (C60). There are other forms of carbon such as coke, charcoal and lamp black which are referred to as amorphous carbon. Coke is the residue left in the conversion of coal to coal gas. Charcoal is obtained when wood or similar vegetable matter is heated in the complete absence o ...
... Buckminster fullerene (C60). There are other forms of carbon such as coke, charcoal and lamp black which are referred to as amorphous carbon. Coke is the residue left in the conversion of coal to coal gas. Charcoal is obtained when wood or similar vegetable matter is heated in the complete absence o ...
chapter15-burno.1348..
... equilibrium constant (KC or Kp) has a particular numerical value. This means that no matter what the starting concentrations or partial pressures for the system, at equilibrium the ratio of the concentrations (partial pressures) of the products divided by the concentrations (partial pressures) of th ...
... equilibrium constant (KC or Kp) has a particular numerical value. This means that no matter what the starting concentrations or partial pressures for the system, at equilibrium the ratio of the concentrations (partial pressures) of the products divided by the concentrations (partial pressures) of th ...
chem A exercise package C
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
Radiation Chemistry of Overirradiated Aqueous Solutions of
... (Reeves 1979), the radiogenic heat o f which could be sufficient to maintain liquid water cores in larger comets for several million years (Irvine et al. 1980; Wallis 1980). This article concerns the effects of irradiation at absorbed doses larger, by up to about one order of magnitude, than those p ...
... (Reeves 1979), the radiogenic heat o f which could be sufficient to maintain liquid water cores in larger comets for several million years (Irvine et al. 1980; Wallis 1980). This article concerns the effects of irradiation at absorbed doses larger, by up to about one order of magnitude, than those p ...
Solubility and Solubility Equilibrium
... When you write out the overall rxn, make sure you write out the net ionic equation (only include ions that do something, spectator ions algebraically cancel out). For the example, the net ionic equation would be: + SO 2BaSO Ba2+ (aq) ...
... When you write out the overall rxn, make sure you write out the net ionic equation (only include ions that do something, spectator ions algebraically cancel out). For the example, the net ionic equation would be: + SO 2BaSO Ba2+ (aq) ...
Calculations Booklet
... Enthalpy of solution of a substance is the energy change when one mole of that substance dissolves in excess water. Enthalpy of solution may be exothermic or endothermic. Worked Example (Note: the method is not always identical) 4g of ammonium nitrate, NH4NO3, is dissolved completely in 100cm3 water ...
... Enthalpy of solution of a substance is the energy change when one mole of that substance dissolves in excess water. Enthalpy of solution may be exothermic or endothermic. Worked Example (Note: the method is not always identical) 4g of ammonium nitrate, NH4NO3, is dissolved completely in 100cm3 water ...