Experiment 22
... produces a shift to the right. This is all true because Kc does not change (unless you change the temperature). The changes in concentration that one can produce by adding particular reagents may be simply enormous, so the shifts in the equilibrium system may also be enormous. Much of the mystery of ...
... produces a shift to the right. This is all true because Kc does not change (unless you change the temperature). The changes in concentration that one can produce by adding particular reagents may be simply enormous, so the shifts in the equilibrium system may also be enormous. Much of the mystery of ...
Prep UK-intro.p65
... The aim of the scientific committee has been that as many as possible of the preparatory problems should take their starting point in issues of general chemical, public or environmental interest. Therefore some of the problems cover several topics from the International Chemistry Olympiad. We have a ...
... The aim of the scientific committee has been that as many as possible of the preparatory problems should take their starting point in issues of general chemical, public or environmental interest. Therefore some of the problems cover several topics from the International Chemistry Olympiad. We have a ...
pdfCfE Higher - Unit 3 - Pupil Booklet 2 MB
... zero but the aim is to learn from mistakes and reduce the rate to a minimum. It is essential at this stage to revise all of your National 5 calculations that were based on moles and equations. ...
... zero but the aim is to learn from mistakes and reduce the rate to a minimum. It is essential at this stage to revise all of your National 5 calculations that were based on moles and equations. ...
Student Review Packet
... container and allowed to reach equilibrium at 1,000 K. Determine whether the equilibrium concentration of HI(g) will be greater than, equal to, or less than the initial concentration of HI(g). Justify your answer. 2004B #1 N2(g) + 3 H2(g) 2 NH3(g) For the reaction represented above, the value of ...
... container and allowed to reach equilibrium at 1,000 K. Determine whether the equilibrium concentration of HI(g) will be greater than, equal to, or less than the initial concentration of HI(g). Justify your answer. 2004B #1 N2(g) + 3 H2(g) 2 NH3(g) For the reaction represented above, the value of ...
QA1
... The wire is then ready to be used. If a sample solution is being tested, immerse the wire into the solution and then put the wire into a colourless flame and note the colour of the flame. Caution should be made not to burn the glass part of the platinum wire; otherwise, it will be broken. Sodium com ...
... The wire is then ready to be used. If a sample solution is being tested, immerse the wire into the solution and then put the wire into a colourless flame and note the colour of the flame. Caution should be made not to burn the glass part of the platinum wire; otherwise, it will be broken. Sodium com ...
CFE Higher Chemistry in Society Homework EB
... Ammonia reduces copper(II) oxide to copper. The other products of the reaction are water and nitrogen. 2NH3 + 3CuO 3Cu + H2O + N2 Calculate the mass of copper produced and the mass of ammonia consumed when 56.4g of copper(II) oxide are reduced in this way. ...
... Ammonia reduces copper(II) oxide to copper. The other products of the reaction are water and nitrogen. 2NH3 + 3CuO 3Cu + H2O + N2 Calculate the mass of copper produced and the mass of ammonia consumed when 56.4g of copper(II) oxide are reduced in this way. ...
Solution Definition and Speciation Calculations
... pe = 16.9Eh, Eh platinum electrode measurement ...
... pe = 16.9Eh, Eh platinum electrode measurement ...
chem 13 news 2010 - University of Waterloo
... its ground electronic state? The atomic number of manganese is Z = 25. ...
... its ground electronic state? The atomic number of manganese is Z = 25. ...
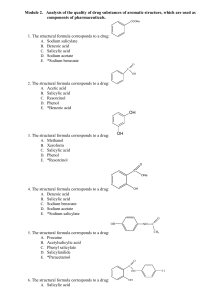
Module 2. Drug substances of aromatic structure
... E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. Phenylsalicylate B. Phthalic acid C. Benzol D. Phenol E. *3-Metylphenol (m-cresol) 83. For assay of resorcinol, according to Pharmacopoeia, use method: A. Cerymetry, direc ...
... E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. Phenylsalicylate B. Phthalic acid C. Benzol D. Phenol E. *3-Metylphenol (m-cresol) 83. For assay of resorcinol, according to Pharmacopoeia, use method: A. Cerymetry, direc ...
NH 4 1+
... Now let’s look at the second reason a double replacement reaction might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and wea ...
... Now let’s look at the second reason a double replacement reaction might occur: the formation of a weak acid. An acid is a compound that has an H+ ion bonded to some negative ion: HNO3 for example is nitric acid. HF is hydrofluoric acid. All acids fall into one of two categories: strong acids and wea ...
AP Chemistry Summer Assignment
... learn more about. The class will be challenging, but the biggest factor in determining your success will be the amount of effort you put into the class. If you do the reading assignments and homework, you can definitely be successful in the class and ultimately on the AP exam. We have a lot of mater ...
... learn more about. The class will be challenging, but the biggest factor in determining your success will be the amount of effort you put into the class. If you do the reading assignments and homework, you can definitely be successful in the class and ultimately on the AP exam. We have a lot of mater ...
1994 Released Exam
... Use your time effectively, workingasrapidlyas you canwithoutlosingaccuracy.Do not spendtoo muchtimeon questionsthatare too difficult. Go on to otherquestions andcomebackto the difficult oneslaterif you havetime. It is not expectedthateveryonewill be ableto answerall the multiple-choicequestions. ...
... Use your time effectively, workingasrapidlyas you canwithoutlosingaccuracy.Do not spendtoo muchtimeon questionsthatare too difficult. Go on to otherquestions andcomebackto the difficult oneslaterif you havetime. It is not expectedthateveryonewill be ableto answerall the multiple-choicequestions. ...
Mechanistic Studies of the Reactions of Silicon
... basis of spectroscopic data, after isolation by semipreparative gas chromatography (GC). The same products were obtained from photolyses in acetonitrile solution, along with small amounts of 9a due to the presence of residual water in the solvent. In the absence of a trapping reagent, photolysis of ...
... basis of spectroscopic data, after isolation by semipreparative gas chromatography (GC). The same products were obtained from photolyses in acetonitrile solution, along with small amounts of 9a due to the presence of residual water in the solvent. In the absence of a trapping reagent, photolysis of ...
Periodic Table and the Atom Answers
... stoichiometry problems, I would highly suggest consulting this section of the site before answering these questions. When doing stoichiometry problems, people are frequently worried by statements such as “if you have an excess of (compound X)”. This statement shouldn’t worry you… what it really mean ...
... stoichiometry problems, I would highly suggest consulting this section of the site before answering these questions. When doing stoichiometry problems, people are frequently worried by statements such as “if you have an excess of (compound X)”. This statement shouldn’t worry you… what it really mean ...
Kinetics Simulations of the Neutralizing Capacity of Silicate Minerals
... In Eq. (6), (A/M) is the ratio of mineral surface area to 1 kg H2O, which is the convention used by PHREEQC, kH, kW, and kOH are rate constants for acidic, neutral, and alkaline conditions, and a and b are the dependencies on H+ and OH- activities, respectively. Table 1 contains a complete list of ...
... In Eq. (6), (A/M) is the ratio of mineral surface area to 1 kg H2O, which is the convention used by PHREEQC, kH, kW, and kOH are rate constants for acidic, neutral, and alkaline conditions, and a and b are the dependencies on H+ and OH- activities, respectively. Table 1 contains a complete list of ...
Chemistry Review - Hicksville Public Schools
... 2. An electrolyte is a substance which, when dissolved in water, forms a solution capable of conducting electricity. The ability to conduct electricity depends on the concentration of ions. 3. Arrhenius acids yield H+(aq) ions as the only positive ion in solution. H+(aq) ions may also be written a ...
... 2. An electrolyte is a substance which, when dissolved in water, forms a solution capable of conducting electricity. The ability to conduct electricity depends on the concentration of ions. 3. Arrhenius acids yield H+(aq) ions as the only positive ion in solution. H+(aq) ions may also be written a ...