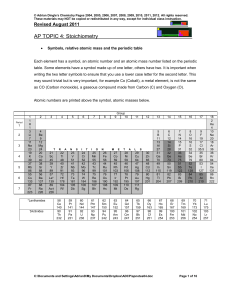
practical identification of organic compounds.docx
... ether. This includes the lower members of the various homologous series (4 5 atoms in a normal chain) that contain oxygen and / or nitrogen in their structures : they are soluble in water because of their low carbon content. If the compound is soluble in both water and in ether, it would also be sol ...
... ether. This includes the lower members of the various homologous series (4 5 atoms in a normal chain) that contain oxygen and / or nitrogen in their structures : they are soluble in water because of their low carbon content. If the compound is soluble in both water and in ether, it would also be sol ...
F325 How Far How Fast test
... 1, 2-Dibromoethane, C2H4Br2, reacts with potassium iodide as shown in the equation ...
... 1, 2-Dibromoethane, C2H4Br2, reacts with potassium iodide as shown in the equation ...
analytical chemistry - Львівський національний медичний
... 4) analysis in drops on filter paper – reaction between analysed substance and analytical reagent run on filter paper with some drops (1-2) of solutions – arise a coloured spots. Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction mus ...
... 4) analysis in drops on filter paper – reaction between analysed substance and analytical reagent run on filter paper with some drops (1-2) of solutions – arise a coloured spots. Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction mus ...
2 - mrstorie
... reaction, how can we minimize the formation of this gas? Explain using your knowledge of Le Chatelier’s principles. Increase the pressure – system will work to decrease the pressure by reducing the number of particles present in the container – shift left to use 2 products to make 1 reactant. 8. Con ...
... reaction, how can we minimize the formation of this gas? Explain using your knowledge of Le Chatelier’s principles. Increase the pressure – system will work to decrease the pressure by reducing the number of particles present in the container – shift left to use 2 products to make 1 reactant. 8. Con ...
uplift luna ap chemistry
... CnH2n+1OH; Do not be fooled—this looks like a hydroxide ion, but is not! It does not make this hydrocarbon an alkaline or basic compound. Do not name these as a hydroxide! C2H6 is ethane while C2H5OH is ethanol. NAMING BINARY IONIC COMPOUNDS How do I know it is ionic? The chemical formula will begin ...
... CnH2n+1OH; Do not be fooled—this looks like a hydroxide ion, but is not! It does not make this hydrocarbon an alkaline or basic compound. Do not name these as a hydroxide! C2H6 is ethane while C2H5OH is ethanol. NAMING BINARY IONIC COMPOUNDS How do I know it is ionic? The chemical formula will begin ...
Theoretical Competition - Austrian Chemistry Olympiad
... June 15th, 2012 The kinetics of gas reactions are usually followed by measuring the total pressure of the gas mixture, which changes with time. In this case the partial pressure of oxygen derives in the following way from the total pressure: ...
... June 15th, 2012 The kinetics of gas reactions are usually followed by measuring the total pressure of the gas mixture, which changes with time. In this case the partial pressure of oxygen derives in the following way from the total pressure: ...
Stoichiometry, Lab Basics, Reactions
... hydrogen gas. The H2 produced was then collected by water displacement at 27C (where the vapor pressure of water is 21 torr) and a barometric pressure of 757 torr. If 0.555 L of gas is collected, the partial pressure of hydrogen gas is: A) 0.555 x (273+27) B) 0.555 x 757 C) 757 – 27 D) 757 – 21 E) ...
... hydrogen gas. The H2 produced was then collected by water displacement at 27C (where the vapor pressure of water is 21 torr) and a barometric pressure of 757 torr. If 0.555 L of gas is collected, the partial pressure of hydrogen gas is: A) 0.555 x (273+27) B) 0.555 x 757 C) 757 – 27 D) 757 – 21 E) ...
Chapter 7: Solutions
... or dissolved NaCl, requires that solute particles be able to interact with the solvent molecules through noncovalent interactions. ...
... or dissolved NaCl, requires that solute particles be able to interact with the solvent molecules through noncovalent interactions. ...
Triple Award - Cheltenham College
... Explain why substances with simple molecular structures have low melting points in terms of the relatively weak forces between the molecules. Explain the high melting points of substances with giant covalent ...
... Explain why substances with simple molecular structures have low melting points in terms of the relatively weak forces between the molecules. Explain the high melting points of substances with giant covalent ...
handout 4
... Try this example on your own to review: During physical activity, lactic acid (molar mass = 90.08 g/mol) forms in muscles and is responsible for muscle soreness. Elemental analysis shows that this compound contains 40.0 mass % C and 53.3 mass % O. Determine the empirical formula and the molecular fo ...
... Try this example on your own to review: During physical activity, lactic acid (molar mass = 90.08 g/mol) forms in muscles and is responsible for muscle soreness. Elemental analysis shows that this compound contains 40.0 mass % C and 53.3 mass % O. Determine the empirical formula and the molecular fo ...
Solved Guess Paper – 3 Q1. Define the term molarity . Ans
... 10. What are non- essential amino acids ? Ans- The amino acids which can be synthesised in the body are known as nonessential amino acids . PART – B 11 a. Mention one consequence of metal excess defect . b. Give an example for molecular solid . Ans- a. Metal excess defect is due to anionic vacancies ...
... 10. What are non- essential amino acids ? Ans- The amino acids which can be synthesised in the body are known as nonessential amino acids . PART – B 11 a. Mention one consequence of metal excess defect . b. Give an example for molecular solid . Ans- a. Metal excess defect is due to anionic vacancies ...
1. Bromine exists naturally as a mixture of bromine
... Analysis shows the compound to be 80% X by mass, with three times as many hydrogen atoms as X atoms per molecule. Which element is element X? A) He B) C C) F D) S E) none of these ...
... Analysis shows the compound to be 80% X by mass, with three times as many hydrogen atoms as X atoms per molecule. Which element is element X? A) He B) C C) F D) S E) none of these ...
national 5 chemistry
... SnO2 in the following reaction: SnO2 + 2H2 Sn + 2H2O 2. Calculate the mass of iron produced from 10g of iron (III) oxide in the following reaction: 2Al + Fe2O3 2Fe + Al2O3 3. What mass of carbon dioxide is formed when 64g of methane burns completely in air? 4. Calculate the mass of ethanol, C2H5 ...
... SnO2 in the following reaction: SnO2 + 2H2 Sn + 2H2O 2. Calculate the mass of iron produced from 10g of iron (III) oxide in the following reaction: 2Al + Fe2O3 2Fe + Al2O3 3. What mass of carbon dioxide is formed when 64g of methane burns completely in air? 4. Calculate the mass of ethanol, C2H5 ...
CLASSES AND NOMENCLATURE OF INORGANIC COMPOUNDS
... 20. What is the electronic configuration of valence electrons corresponds to an element of 4th period of the VI group of main sub-group: A 4s2 4p4 B 4s1 3d5 C 6s2 6p2 D 6s2 5d2 E 3s2 3p4 22. On the base of electronic structure of the atom ...
... 20. What is the electronic configuration of valence electrons corresponds to an element of 4th period of the VI group of main sub-group: A 4s2 4p4 B 4s1 3d5 C 6s2 6p2 D 6s2 5d2 E 3s2 3p4 22. On the base of electronic structure of the atom ...
Concentration of solutions
... and a solution of a non-electrolyte in that solvent, and it is directly proportional to the molal concentration of the solution. ∆Tf = Kf m • Kf = the molal freezing-point constant is the freezing-point depression of the solvent in a 1molal solution of a nonvolatile, nonelectrolyte solute. The value ...
... and a solution of a non-electrolyte in that solvent, and it is directly proportional to the molal concentration of the solution. ∆Tf = Kf m • Kf = the molal freezing-point constant is the freezing-point depression of the solvent in a 1molal solution of a nonvolatile, nonelectrolyte solute. The value ...
Equilibrium - District 196
... • The ratio of the concentrations should also remain constant ***The only stressor that can affect these concentration ratios is temperature ***Keq is temperature dependant ...
... • The ratio of the concentrations should also remain constant ***The only stressor that can affect these concentration ratios is temperature ***Keq is temperature dependant ...
Part II - American Chemical Society
... need not balance the equations. All reactions occur in aqueous solution unless otherwise indicated. a. Excess carbon dioxide is bubbled through a suspension of calcium hydroxide. b. Acidified solutions of cerium(IV) and iron(II) are mixed. c. Solid calcium carbide is added to water. d. Excess concen ...
... need not balance the equations. All reactions occur in aqueous solution unless otherwise indicated. a. Excess carbon dioxide is bubbled through a suspension of calcium hydroxide. b. Acidified solutions of cerium(IV) and iron(II) are mixed. c. Solid calcium carbide is added to water. d. Excess concen ...