Atoms, molecules and ions
... Atomic number, Atomic Mass, and Isotopes • Atomic number: The number of protons in the nucleus of each atom of a given element • Atomic mass: The total number of neutrons and protons contained in the nucleus of an atom • All atoms of an element have the same number of protons, but not necessarily t ...
... Atomic number, Atomic Mass, and Isotopes • Atomic number: The number of protons in the nucleus of each atom of a given element • Atomic mass: The total number of neutrons and protons contained in the nucleus of an atom • All atoms of an element have the same number of protons, but not necessarily t ...
Acid Base Equilibria
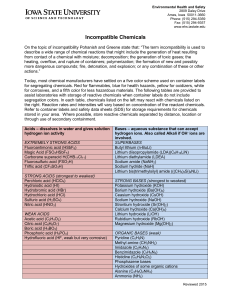
... Weak acid: one that only partially dissociates in aqueous solution and therefore exists in the solution as a mixture of acid molecules component ions: For example, HF dissociates in water to give H+ and F-. It is a weak acid with a dissociation equation that is HF (aq) ↔ H+ (aq) + F-(aq) Note the us ...
... Weak acid: one that only partially dissociates in aqueous solution and therefore exists in the solution as a mixture of acid molecules component ions: For example, HF dissociates in water to give H+ and F-. It is a weak acid with a dissociation equation that is HF (aq) ↔ H+ (aq) + F-(aq) Note the us ...
makeup6
... 10. Which of the following molecules contains a central atom which is sp2 hybridized? (A) H2SO4 (B) H2CO3 (C) ICl2 (D) H3CCH3 11. Which of the following molecules has at least one non-bonding pair of electrons on the central atom? (A) CHCl3 (B) HCN (C) H2CO (D) O3 12. Which of the following statemen ...
... 10. Which of the following molecules contains a central atom which is sp2 hybridized? (A) H2SO4 (B) H2CO3 (C) ICl2 (D) H3CCH3 11. Which of the following molecules has at least one non-bonding pair of electrons on the central atom? (A) CHCl3 (B) HCN (C) H2CO (D) O3 12. Which of the following statemen ...
Semester Exam Review
... (c) A 0.345 g sample of anhydrous BeC2O4, which contains an inert impurity, was dissolved in sufficient water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4(aq). The balanced equation for the reaction that occurred is as follows. 16 H+(aq) + 2 MnO4-(aq) + 5 ...
... (c) A 0.345 g sample of anhydrous BeC2O4, which contains an inert impurity, was dissolved in sufficient water to produce 100. mL of solution. A 20.0 mL portion of the solution was titrated with KMnO4(aq). The balanced equation for the reaction that occurred is as follows. 16 H+(aq) + 2 MnO4-(aq) + 5 ...
Unit 2: Atoms, Ions and Ionic Compounds
... Write the equilibrium constant expression and its numerical value. Set up a table showing initial concentration, change, equilibrium ...
... Write the equilibrium constant expression and its numerical value. Set up a table showing initial concentration, change, equilibrium ...
Chem 11 Study Guide SCH3U Unit 1 Definitions: SATP: Standard
... These definitions are correct but not general enough to include the wide range of acid and base substances which are known to exist. In addition, they rely on the use of water as a solvent, which is also too narrow. Bronsted-Lowry acids and bases A Bronsted-Lowry (BL) acid is defined as any substanc ...
... These definitions are correct but not general enough to include the wide range of acid and base substances which are known to exist. In addition, they rely on the use of water as a solvent, which is also too narrow. Bronsted-Lowry acids and bases A Bronsted-Lowry (BL) acid is defined as any substanc ...
Fall Exam 4
... is a high-energy transition state that molecules must go through to convert from reactants to products. represents the fraction of molecules that have enough energy to make it over the activation barrier on a given approach. is the energy barrier that must be surmounted for reactants to be transform ...
... is a high-energy transition state that molecules must go through to convert from reactants to products. represents the fraction of molecules that have enough energy to make it over the activation barrier on a given approach. is the energy barrier that must be surmounted for reactants to be transform ...
chemistry important question i
... (b) Which polymer is obtained when free radical polymerisation of chloroprene occurs? Write the structure of the polymer thus obtained. An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms ...
... (b) Which polymer is obtained when free radical polymerisation of chloroprene occurs? Write the structure of the polymer thus obtained. An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms ...
Chapter 13 - "Water and Solutions"
... – Explaining Acid-Base Properties • Acid base properties are explained by looking at how they ionize in water. • Equilibrium occurs when two opposing reactions happen at the same time and rate. – H2O + H2O H3O+ + OH• This reaction can occur in both directions and chemical equilibrium is reached w ...
... – Explaining Acid-Base Properties • Acid base properties are explained by looking at how they ionize in water. • Equilibrium occurs when two opposing reactions happen at the same time and rate. – H2O + H2O H3O+ + OH• This reaction can occur in both directions and chemical equilibrium is reached w ...
2012 Coaches Institute Presentation
... The percentage of acid molecules that ionize in water is another measure of the strength of an acid % Ionization = M(ionized acid) x ...
... The percentage of acid molecules that ionize in water is another measure of the strength of an acid % Ionization = M(ionized acid) x ...
Lecture notes Chapters 10
... Saponification: Natural soap are sodium or potassium salts of fatty acids. They are prepared from a blend of tallow and coconut oils (triglyceride). Triglycerides are triesters of glycerol. The solid fats are melted with steam, and the water insoluble triglyceride layer that forms on the top is remo ...
... Saponification: Natural soap are sodium or potassium salts of fatty acids. They are prepared from a blend of tallow and coconut oils (triglyceride). Triglycerides are triesters of glycerol. The solid fats are melted with steam, and the water insoluble triglyceride layer that forms on the top is remo ...
Unit 2: Atoms and Ions Homework Booklet
... 3. Read the following passage and list the raw materials and their products in the form of a table. It’s quite surprising what chemists do! They make useful substances like soaps and bleach from raw materials such as sea water. Crude oil which is a sticky black mixture, can be manufactured into lubr ...
... 3. Read the following passage and list the raw materials and their products in the form of a table. It’s quite surprising what chemists do! They make useful substances like soaps and bleach from raw materials such as sea water. Crude oil which is a sticky black mixture, can be manufactured into lubr ...
Acids, Bases, and pH
... You may have heard that cola drinks are acidic, but what about regular carbonated water with no other additives? It turns out that carbonated water has a slightly lower pH than still (not carbonated) water. What does it mean to have a low pH? How is pH measured and how does it relate to the acidity ...
... You may have heard that cola drinks are acidic, but what about regular carbonated water with no other additives? It turns out that carbonated water has a slightly lower pH than still (not carbonated) water. What does it mean to have a low pH? How is pH measured and how does it relate to the acidity ...
S3 Chemistry - eduBuzz.org
... State the location, charge and mass of each sub atomic particle Calculate the number of protons, neutrons and electrons in an atom Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion ...
... State the location, charge and mass of each sub atomic particle Calculate the number of protons, neutrons and electrons in an atom Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion ...
Learning Outcomes for Chemical Reactions and
... • State the location, charge and mass of each sub atomic particle • Calculate the number of protons, neutrons and electrons in an atom • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion ...
... • State the location, charge and mass of each sub atomic particle • Calculate the number of protons, neutrons and electrons in an atom • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion ...
Bio Review note stems
... This chapter is a review from your previous biology class – these concepts are critical and repeated throughout the year. If you have not covered this material previously or need additional assistance with the concepts please schedule time to see me. 1. Why is water considered a polar molecule? 2. F ...
... This chapter is a review from your previous biology class – these concepts are critical and repeated throughout the year. If you have not covered this material previously or need additional assistance with the concepts please schedule time to see me. 1. Why is water considered a polar molecule? 2. F ...
Oxidation-Reduction Reactions
... Acid: proton donor, Base: proton acceptor – The Arrhenius concept acid: proton (H+) donor base: hydroxide ion (OH-) donor In summary, both concepts the same ...
... Acid: proton donor, Base: proton acceptor – The Arrhenius concept acid: proton (H+) donor base: hydroxide ion (OH-) donor In summary, both concepts the same ...