Organic Compounds
... – Carbon: normally forms four covalent bonds and has no unshared pairs of electrons. C – Hydrogen: forms one covalent bond and no unshared pairs of electrons. H – Nitrogen: normally forms three covalent bonds and has one unshared pair of electrons. ...
... – Carbon: normally forms four covalent bonds and has no unshared pairs of electrons. C – Hydrogen: forms one covalent bond and no unshared pairs of electrons. H – Nitrogen: normally forms three covalent bonds and has one unshared pair of electrons. ...
Recall
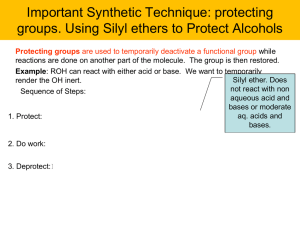
... groups. Using Silyl ethers to Protect Alcohols Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily ...
... groups. Using Silyl ethers to Protect Alcohols Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily ...
HILL12_Lecture_09
... maximum number of hydrogen atoms attached to each carbon and no double or triple bonds. Unsaturated hydrocarbons can undergo an addition reaction, such as that seen on the right here. © 2010 Pearson Prentice Hall, Inc. ...
... maximum number of hydrogen atoms attached to each carbon and no double or triple bonds. Unsaturated hydrocarbons can undergo an addition reaction, such as that seen on the right here. © 2010 Pearson Prentice Hall, Inc. ...
Unsaturated Hydrocarbons I : Alkenes
... Unsaturated Hydrocarbons 1 Alkenes By: Dr. Siham Lahsasni ...
... Unsaturated Hydrocarbons 1 Alkenes By: Dr. Siham Lahsasni ...
An Epoxidation Reaction: The Epoxidation of Cholesterol to 5 ,6
... Figure 1. Structures of water, alcohols and ethers. Esters are acid derivatives and contain a carbonyl group; whereas, ethers are water or alcohol derivatives and do not contain a carbonyl group. Alcohols are named by finding the longest carbon chain to which the OH group is bonded and naming the al ...
... Figure 1. Structures of water, alcohols and ethers. Esters are acid derivatives and contain a carbonyl group; whereas, ethers are water or alcohol derivatives and do not contain a carbonyl group. Alcohols are named by finding the longest carbon chain to which the OH group is bonded and naming the al ...
Isomers
... much higher melting point. Unlike the cis isomer there is little intra-molecular hydrogen bonding ...
... much higher melting point. Unlike the cis isomer there is little intra-molecular hydrogen bonding ...
Chemical bonding and structure
... as protons and electrons. This is because the number of protons (+) is equal to the number of electrons (−), and so their charges cancel each other out. The positively charged protons, located within the nucleus of the atom, are not transferred during chemical reactions. Electrons, however, position ...
... as protons and electrons. This is because the number of protons (+) is equal to the number of electrons (−), and so their charges cancel each other out. The positively charged protons, located within the nucleus of the atom, are not transferred during chemical reactions. Electrons, however, position ...
7. Organic halides
... Conformation and reactivity of cycloalkanes Experimental observations show that cyclopropane (and, to a lesser extent, cyclobutane) differ in reactivity from the larger cycloalkanes and acyclic alkanes. Cyclopropane exhibits easy ring opening (see p. 20) instead of substitution characteristic of alk ...
... Conformation and reactivity of cycloalkanes Experimental observations show that cyclopropane (and, to a lesser extent, cyclobutane) differ in reactivity from the larger cycloalkanes and acyclic alkanes. Cyclopropane exhibits easy ring opening (see p. 20) instead of substitution characteristic of alk ...
L1 - Amines
... • Organic bases. • Generally have strong, unpleasant odors. • Are found extensively in biological systems. • Found in both controlled and medicinal compounds 1,5 - diaminopentane H2N – CH2-CH2-CH2-CH2-CH2 – NH2 Cadaverine ...
... • Organic bases. • Generally have strong, unpleasant odors. • Are found extensively in biological systems. • Found in both controlled and medicinal compounds 1,5 - diaminopentane H2N – CH2-CH2-CH2-CH2-CH2 – NH2 Cadaverine ...
Halogenoalkanes
... Note that this reaction is very exothermic so the solution must be cold, or dry ice (solid CO2 at –78o C used instead). ...
... Note that this reaction is very exothermic so the solution must be cold, or dry ice (solid CO2 at –78o C used instead). ...
Some more basic organic (more naming, reactions, polymers)
... pleasant odors. The most important compound in this family is benzene. ...
... pleasant odors. The most important compound in this family is benzene. ...
H - Knockhardy
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
No Slide Title
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
... This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purp ...
Structural Effects on Acidity
... Contribution due to intramolecular H-bonding is not significant in the o- nitrophenol, thus no apparent differences in ka when nitro group is placed in the o or p position. The greater acidity of the o and p nitrophenols as compared with the meta is attributed to the stronger effect of pi electron d ...
... Contribution due to intramolecular H-bonding is not significant in the o- nitrophenol, thus no apparent differences in ka when nitro group is placed in the o or p position. The greater acidity of the o and p nitrophenols as compared with the meta is attributed to the stronger effect of pi electron d ...
Chapter 10 Chemical Bonding Theories
... Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
... Bonds form using shared electrons between overlapping orbitals on adjacent atoms. Orbitals arrange around central atom to avoid each other. Two types of bonds: sigma and pi. ...
Organic Chemistry Notes
... If a double bond is present, change the “ane” ending to “ene” If a triple bond is present, change the “ane” ending to “yne” The bond number goes from the lower numbered carbon to the higher numbered one. The number goes immediately in front of the name of the parent hydrocarbon, separated by a ...
... If a double bond is present, change the “ane” ending to “ene” If a triple bond is present, change the “ane” ending to “yne” The bond number goes from the lower numbered carbon to the higher numbered one. The number goes immediately in front of the name of the parent hydrocarbon, separated by a ...
CH 3
... formula (also known as a bond-line formula or carbon skeleton diagram). • In skeletal formulae, carbon atoms are not signified by the symbol C but by the vertices of the lines. • Hydrogen atoms bonded to carbon are not shown — they can be inferred by counting the number of bonds to a particular carb ...
... formula (also known as a bond-line formula or carbon skeleton diagram). • In skeletal formulae, carbon atoms are not signified by the symbol C but by the vertices of the lines. • Hydrogen atoms bonded to carbon are not shown — they can be inferred by counting the number of bonds to a particular carb ...
on nomenclature. compounds other than hydrocarbons%
... "The reason for giving multiple suffixes for some groups will become clearer later. The basic idea is that we use pentanoic acid for CH,CH,CH,CH2C02H but cyclobutanecarboxylic acid for ()-CO,H. In the first case, the -CO,H carbon is part of the chain, but it is not in the second. bAlcohols and pheno ...
... "The reason for giving multiple suffixes for some groups will become clearer later. The basic idea is that we use pentanoic acid for CH,CH,CH,CH2C02H but cyclobutanecarboxylic acid for ()-CO,H. In the first case, the -CO,H carbon is part of the chain, but it is not in the second. bAlcohols and pheno ...
Year 1 Foundation course, section B2
... Year 1 Foundation course, section B2; Structure and reactivity of specific functional groups Alkanes - the most basic of all organic compounds, composed of only C and H, with no functional groups. General formulae CnH2n+2 (unless cyclic in which case it is CnH2n). Alkanes are generally quite unreac ...
... Year 1 Foundation course, section B2; Structure and reactivity of specific functional groups Alkanes - the most basic of all organic compounds, composed of only C and H, with no functional groups. General formulae CnH2n+2 (unless cyclic in which case it is CnH2n). Alkanes are generally quite unreac ...
Organic Chemistry - Madison Public Schools
... Biological Chemistry Organic and Biological Chemistry ...
... Biological Chemistry Organic and Biological Chemistry ...
Physical Organic Chemistry
... halides are less reactive in Nucleophilic substitution reaction due to: high electron density in benzene ring. bond in C-X stronger and shorter Aryl cation unstable therefore no SN1 There is no transition state with same plane of the ring C-Br hence no SN2 ...
... halides are less reactive in Nucleophilic substitution reaction due to: high electron density in benzene ring. bond in C-X stronger and shorter Aryl cation unstable therefore no SN1 There is no transition state with same plane of the ring C-Br hence no SN2 ...
01. Introduction of bioorganic chemistry. Classification, structure
... with one hydrogen atom bonded to each carbon atom and with three double carbon-carbon bonds. Benzene doesn’t react as a typical alkene (doesn’t decolorize bromine solution, negative Bayer’s test). Benzene behaves chemically like a typical alkane (substitution reactions). ...
... with one hydrogen atom bonded to each carbon atom and with three double carbon-carbon bonds. Benzene doesn’t react as a typical alkene (doesn’t decolorize bromine solution, negative Bayer’s test). Benzene behaves chemically like a typical alkane (substitution reactions). ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.