Haloalkanes and Haloarenes
... The reactions of primary and secondary alcohols with HCl require the presence of a catalyst, ZnCl2. With tertiary alcohols, the reaction is conducted by simply shaking with concentrated HCl at room temperature. Constant boiling with HBr (48%) is used for preparing alkyl bromide. Good yields of R—I m ...
... The reactions of primary and secondary alcohols with HCl require the presence of a catalyst, ZnCl2. With tertiary alcohols, the reaction is conducted by simply shaking with concentrated HCl at room temperature. Constant boiling with HBr (48%) is used for preparing alkyl bromide. Good yields of R—I m ...
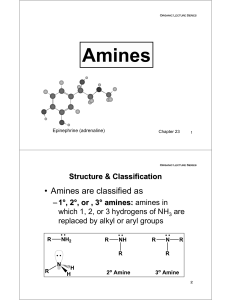
Amines
... 1° RNH2 with HNO2 • Aliphatic diazonium ions are unstable and lose N2 to give a carbocation which may 1. lose a proton to give an alkene 2. react with a nucleophile to give a substitution product 3. rearrange and then react by 1 and/or 2 ...
... 1° RNH2 with HNO2 • Aliphatic diazonium ions are unstable and lose N2 to give a carbocation which may 1. lose a proton to give an alkene 2. react with a nucleophile to give a substitution product 3. rearrange and then react by 1 and/or 2 ...
Part 2 - Class Index
... Name the main chain (or ring), numbering the C=C or CC bonds if necessary. If this gives a name in which the next letter after the ‘e’ of ‘ane’, ‘ene’ or ‘yne’ is a vowel, drop the ‘e’. -3-penten-2-one Name and number the substituents on the main chain. If a substituent appears more than once, use ...
... Name the main chain (or ring), numbering the C=C or CC bonds if necessary. If this gives a name in which the next letter after the ‘e’ of ‘ane’, ‘ene’ or ‘yne’ is a vowel, drop the ‘e’. -3-penten-2-one Name and number the substituents on the main chain. If a substituent appears more than once, use ...
Chapter 11 Lecture Notes: Alcohols, Ethers, Aldehydes, and Ketones
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...
Chapter 8 Alkenes and Alkynes II
... Product with the more stable carbocation intermediate predominat es Transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations ...
... Product with the more stable carbocation intermediate predominat es Transition state for the rate determining step (first step) resembles a carbocation and is stabilized by factors which stabilize carbocations ...
Dipole Moment
... Distances between the neighboring atoms of the two functional groups (X-H- - -Y) in hydrogen bonds are substantially smaller than the sum of their van der Waals radii. X--H stretching modes are shifted toward lower frequencies (lower ...
... Distances between the neighboring atoms of the two functional groups (X-H- - -Y) in hydrogen bonds are substantially smaller than the sum of their van der Waals radii. X--H stretching modes are shifted toward lower frequencies (lower ...
CH 2 CH(CH 3 ) - Parkway C-2
... Always include both the double and triple bond in the longest chain – even if it isn’t the most number of carbons! Start counting from the end closest to the double or triple bond – whichever has the lowest number If there is a tie, and only then, do double bonds take priority over triple ...
... Always include both the double and triple bond in the longest chain – even if it isn’t the most number of carbons! Start counting from the end closest to the double or triple bond – whichever has the lowest number If there is a tie, and only then, do double bonds take priority over triple ...
Basic definitions for organic chemistry
... • addition reactions will have 100% atom economy • substitution reactions will have less than 100% atom economy • elimination reactions will have less than 100% atom economy • high atom economy = fewer waste materials • reactions may have a high yield but a low atom economy ...
... • addition reactions will have 100% atom economy • substitution reactions will have less than 100% atom economy • elimination reactions will have less than 100% atom economy • high atom economy = fewer waste materials • reactions may have a high yield but a low atom economy ...
M.Sc - I Syllabus w.e.f. June 2015
... Life time of electronically excited state, electronic transition and intensity of absorption bands, Construction of Jablonski diagram, Photophysical pathways of excited molecular systems, Fluorescence and phosphorescence, Stern-Volmer relation, critical energy transfer distances, energy transfer eff ...
... Life time of electronically excited state, electronic transition and intensity of absorption bands, Construction of Jablonski diagram, Photophysical pathways of excited molecular systems, Fluorescence and phosphorescence, Stern-Volmer relation, critical energy transfer distances, energy transfer eff ...
Organomet-2
... empty metal d-orbitals and backdonation from filled metal dorbitals to * orbitals - synergic bonding (H/S p 588) Most stable alkene complexes are found with metals in low ...
... empty metal d-orbitals and backdonation from filled metal dorbitals to * orbitals - synergic bonding (H/S p 588) Most stable alkene complexes are found with metals in low ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... and the Greek ‘eidos’ (type, similarity). ...
... and the Greek ‘eidos’ (type, similarity). ...
structure and substitution. Thus, the culties associated with
... groups resonate at lower field than do carbons attached to nitrogen ...
... groups resonate at lower field than do carbons attached to nitrogen ...
13C NMR Spectroscopy (#1d)
... C NMR spectra cover a wide range, from 0 to about 220 ppm, where carbonyl carbons are found. The degree of substitution on a carbon has about as much effect on its chemical shift position as does the presence of an electronegative atom. Generally, sp3-hybridized carbons appear between 8-60 ppm and s ...
... C NMR spectra cover a wide range, from 0 to about 220 ppm, where carbonyl carbons are found. The degree of substitution on a carbon has about as much effect on its chemical shift position as does the presence of an electronegative atom. Generally, sp3-hybridized carbons appear between 8-60 ppm and s ...
Uranyl Ion Complexes with Ammoniobenzoates as
... ammonium substituent. The nitrogen atom N1 is at a distance of 0.082(5) Å from the portal mean plane and it forms hydrogen bonds with the carbonyl atoms O9 and O11, and with the oxygen atom of a DMF molecule included in the CB6 cavity [N···O and H···O distances, and N–H···O angles are in the ranges ...
... ammonium substituent. The nitrogen atom N1 is at a distance of 0.082(5) Å from the portal mean plane and it forms hydrogen bonds with the carbonyl atoms O9 and O11, and with the oxygen atom of a DMF molecule included in the CB6 cavity [N···O and H···O distances, and N–H···O angles are in the ranges ...
Molecular geometry
... Valence bond theory versus molecular orbital theory Valence bond theory (VB): An advanced model of chemical bonding in which electrons reside in quantum-mechanical orbitals localized on individual atoms that are a hybridized blend of standard atomic orbitals; chemical bonds result from an overlap ...
... Valence bond theory versus molecular orbital theory Valence bond theory (VB): An advanced model of chemical bonding in which electrons reside in quantum-mechanical orbitals localized on individual atoms that are a hybridized blend of standard atomic orbitals; chemical bonds result from an overlap ...
Amines
... - the presence of alkyl groups (electron-donating group) such as (CH3) and (CH3CH2-) will make the amine become more basic. - for example, methylamine is more basic than ammonia. ii) substitution by electron-withdrawing groups - the presence of electron-withdrawing groups or atom will decrease the b ...
... - the presence of alkyl groups (electron-donating group) such as (CH3) and (CH3CH2-) will make the amine become more basic. - for example, methylamine is more basic than ammonia. ii) substitution by electron-withdrawing groups - the presence of electron-withdrawing groups or atom will decrease the b ...
Chapter 12- Alcohols from Carbonyl Compounds, Redox Reactions
... • Grignard additions to carbonyl compounds are especially useful because they can be used to prepared primary, secondary, and tertiary alcohols. – Grignards + formaldehyde to get primary alcohols – Grignards + all other aldehydes to get secondary alcohols – Gignards + ketones to get tertiary alcohol ...
... • Grignard additions to carbonyl compounds are especially useful because they can be used to prepared primary, secondary, and tertiary alcohols. – Grignards + formaldehyde to get primary alcohols – Grignards + all other aldehydes to get secondary alcohols – Gignards + ketones to get tertiary alcohol ...
UNSATURATED HYDROCARBONS
... Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. Those with at least one double bond are called alkenes and those with at least one triple bond are called alkynes. Alkenes with two double bonds are called dienes. ...
... Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. Those with at least one double bond are called alkenes and those with at least one triple bond are called alkynes. Alkenes with two double bonds are called dienes. ...
Course Specification Organic Chemistry
... Provide students with basic background in organic and physical organic chemistry To give students skills and abilities needed in the higher years. 3- Intended Learning Outcomes of Course(ILOs): By the end of this cours, the student should be able to: a- Knowledge and Understanding: al- To define the ...
... Provide students with basic background in organic and physical organic chemistry To give students skills and abilities needed in the higher years. 3- Intended Learning Outcomes of Course(ILOs): By the end of this cours, the student should be able to: a- Knowledge and Understanding: al- To define the ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.