Document
... • Organic Chemistry is the study of carboncontaining compounds and their properties. • Oxides and carbonates that contain carbon are not considered to be organic, they are inorganic. • The original distinction between organic and inorganic was based on whether a compound was produced by living thing ...
... • Organic Chemistry is the study of carboncontaining compounds and their properties. • Oxides and carbonates that contain carbon are not considered to be organic, they are inorganic. • The original distinction between organic and inorganic was based on whether a compound was produced by living thing ...
Slide 1
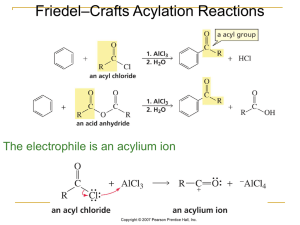
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
Aromatic Compounds
... hexagon. All bond angles are 120º, all carbon atoms are sp2-hybridized, and all carbon-carbon bond lengths are 139 pm • Benzene undergoes substitution reactions that retain the cyclic conjugation rather than electrophilic addition reactions that would destroy the conjugation • Benzene is a resonance ...
... hexagon. All bond angles are 120º, all carbon atoms are sp2-hybridized, and all carbon-carbon bond lengths are 139 pm • Benzene undergoes substitution reactions that retain the cyclic conjugation rather than electrophilic addition reactions that would destroy the conjugation • Benzene is a resonance ...
Gr - loyolascience2
... section of the molecule, as in the case of Vitamin A). This allows p-orbital electrons to become delocalized above and below the plane of the molecule to form “pi-clouds” (the plane of the molecule is formed by sigma bonds). Multiple Names Figure 7 (p. 19) shows the condensed structural formula of t ...
... section of the molecule, as in the case of Vitamin A). This allows p-orbital electrons to become delocalized above and below the plane of the molecule to form “pi-clouds” (the plane of the molecule is formed by sigma bonds). Multiple Names Figure 7 (p. 19) shows the condensed structural formula of t ...
Word - chemmybear.com
... that one way molecules can join, a reaction where H2O is formed, is called a “condensation reaction.” that a polymer (“poly” means many, “mer” means parts) consists of many repeating parts. a second way molecules can join, in which a double bond opens to form two new bonds, is called an “addition re ...
... that one way molecules can join, a reaction where H2O is formed, is called a “condensation reaction.” that a polymer (“poly” means many, “mer” means parts) consists of many repeating parts. a second way molecules can join, in which a double bond opens to form two new bonds, is called an “addition re ...
CAN YOU …
... use IUPAC rules to name and draw alkanes, alkenes, and alkynes identify the physical properties of alkanes identify a functional group name and draw compounds with functional groups such as esters, ethers, aldehydes, alcohols, ketones, haloalkanes, carboxylic acids, amines and….using IUPAC rules des ...
... use IUPAC rules to name and draw alkanes, alkenes, and alkynes identify the physical properties of alkanes identify a functional group name and draw compounds with functional groups such as esters, ethers, aldehydes, alcohols, ketones, haloalkanes, carboxylic acids, amines and….using IUPAC rules des ...
Types of Chemical Reactions
... decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes called single displacement): atoms of one element replace atoms of another in a compound: A + BC AC + B Most often, AC and BC are ionic compounds, which means A and B are metals ...
... decomposition: one reactant disintegrates (decomposes) to form two or more products: AB+C single replacement (sometimes called single displacement): atoms of one element replace atoms of another in a compound: A + BC AC + B Most often, AC and BC are ionic compounds, which means A and B are metals ...
Organic Nomenclature Notes
... CH3 A compound like this has a main chain made up of three carbons. It also has an extra branch with one carbon in it. We name the main chain butane, because it has 4 carbons in it and all the carbon-carbon bonds are single. We name the branch a methyl branch, since there is only one carbon in the b ...
... CH3 A compound like this has a main chain made up of three carbons. It also has an extra branch with one carbon in it. We name the main chain butane, because it has 4 carbons in it and all the carbon-carbon bonds are single. We name the branch a methyl branch, since there is only one carbon in the b ...
CHEMISTRY VOCABULARY
... An ATOM is the smallest part of an element. ELEMENTS cannot be broken down by either physical or chemical methods. In PHYSICAL processes no new substance is made. In CHEMICAL processes something new is made. ATOMS have a nucleus, which contains protons and neutrons, Electrons are arranged around the ...
... An ATOM is the smallest part of an element. ELEMENTS cannot be broken down by either physical or chemical methods. In PHYSICAL processes no new substance is made. In CHEMICAL processes something new is made. ATOMS have a nucleus, which contains protons and neutrons, Electrons are arranged around the ...
Organic Chemistry Unit
... Found in all living matter Found in body tissue Found in food Found in fuels (coal, wood, petroleum) ...
... Found in all living matter Found in body tissue Found in food Found in fuels (coal, wood, petroleum) ...
notes fill in File
... Organic means "____________ _________ ___________" because scientists once thought only "living" organisms could produce organic compounds (they were wrong -- _________________ made urea in 1828…. It is an organic compound) All organic compounds contain carbon but not all carbon containing compounds ...
... Organic means "____________ _________ ___________" because scientists once thought only "living" organisms could produce organic compounds (they were wrong -- _________________ made urea in 1828…. It is an organic compound) All organic compounds contain carbon but not all carbon containing compounds ...
Chapter 2 - Families of Carbon Compounds
... alcohol has the functional group known as a hydroxyl group, −OH, that attaches to an sp3 -hybridized ...
... alcohol has the functional group known as a hydroxyl group, −OH, that attaches to an sp3 -hybridized ...
Exam 3 Key
... For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. The condition of an atom that has at least one of its electrons in orbitals that do not represent the lowest possible potential energy is called a(n) excited state. 2. A(n ...
... For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. The condition of an atom that has at least one of its electrons in orbitals that do not represent the lowest possible potential energy is called a(n) excited state. 2. A(n ...
Chapter 3 – sections 3
... That means that carbon can form long chains, branched chains, or even rings. Many molecules that make up our bodies consist of thousands of carbon atoms bonded together. What kind of bond does carbon form? Covalent – which are strong, sturdy bonds – Because they are stable bonds, they are capable of ...
... That means that carbon can form long chains, branched chains, or even rings. Many molecules that make up our bodies consist of thousands of carbon atoms bonded together. What kind of bond does carbon form? Covalent – which are strong, sturdy bonds – Because they are stable bonds, they are capable of ...
Bonding and Nomenclature
... 2. Predict formulas for stable ionic compounds (binary and tertiary) based on balance of charges. 3. Use IUPAC nomenclature for both chemical names and formulas: •Ionic compounds (Binary and tertiary) •Covalent compounds (Binary and tertiary) 4. Apply concepts of the mole and Avogadro’s number to co ...
... 2. Predict formulas for stable ionic compounds (binary and tertiary) based on balance of charges. 3. Use IUPAC nomenclature for both chemical names and formulas: •Ionic compounds (Binary and tertiary) •Covalent compounds (Binary and tertiary) 4. Apply concepts of the mole and Avogadro’s number to co ...
Chapter 2 - people.vcu.edu
... A molecule can belong to more than one class of compound! I can guarantee that at some point you will be asked to circle the functional groups on a large molecule and state what class of compound each functional group makes the molecule. ...
... A molecule can belong to more than one class of compound! I can guarantee that at some point you will be asked to circle the functional groups on a large molecule and state what class of compound each functional group makes the molecule. ...
Zumd22
... With the exception of petroleum products, geological and elemental carbon is inorganic carbon. Other carbon-containing molecules are organic by virtue of carbon’s presence. There are no end to the combinatorial possibilities since C bonds to C! ...
... With the exception of petroleum products, geological and elemental carbon is inorganic carbon. Other carbon-containing molecules are organic by virtue of carbon’s presence. There are no end to the combinatorial possibilities since C bonds to C! ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.