Practice Problem - HCC Southeast Commons
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
... resonance effects that reinforce each other – The ortho and para intermediates are destabilized – The positive charge of the carbocation intermediate in ortho and para attack is directly on the carbon that bears the deactivating group and resonance ...
Polymers
... molecular formula (although it may sometimes also be the empirical formula). Ex: acetylene is written C2H2 not CH It is often more useful to write out the structural formula which shows in what order the atoms are bonded to each other. Ex: Propane C3H8 ...
... molecular formula (although it may sometimes also be the empirical formula). Ex: acetylene is written C2H2 not CH It is often more useful to write out the structural formula which shows in what order the atoms are bonded to each other. Ex: Propane C3H8 ...
Chapter 1--Title
... If the carbon is attached to one other carbon that carbon is primary (1o) and the alkyl halide is also 1o If the carbon is attached to two other carbons, that carbon is secondary (2 o) and the alkyl halide is 2o If the carbon is attached to three other carbons, the carbon is tertiary (3 o) and the a ...
... If the carbon is attached to one other carbon that carbon is primary (1o) and the alkyl halide is also 1o If the carbon is attached to two other carbons, that carbon is secondary (2 o) and the alkyl halide is 2o If the carbon is attached to three other carbons, the carbon is tertiary (3 o) and the a ...
Section 2.6
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
... • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions ...
Test 12
... Amines and Amides Identify an Amino Acid (has amine group and acid group) Organic Reactions Substitution: with alkanes, one atom substitutes for another, often happens with halogens Addition: with alkenes/alkynes, two atoms add on as a double or triple bond is broken Esterification: alcohol + organi ...
... Amines and Amides Identify an Amino Acid (has amine group and acid group) Organic Reactions Substitution: with alkanes, one atom substitutes for another, often happens with halogens Addition: with alkenes/alkynes, two atoms add on as a double or triple bond is broken Esterification: alcohol + organi ...
ORGANOMETALLIC COMPOUNDS
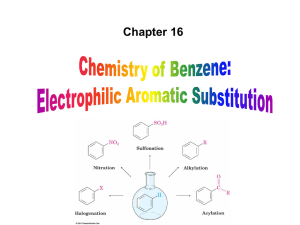
... Synthesis: Friedel‐Crafts reaction. Educts: chromium trichloride, aluminium, benzene, aluminium chloride, sodium sulphate. Process: chromium trichloride reacts with aluminium and benzene with aluminium chloride as catalyst. The buildet complex then reacts with sodium sulphate and forms ...
... Synthesis: Friedel‐Crafts reaction. Educts: chromium trichloride, aluminium, benzene, aluminium chloride, sodium sulphate. Process: chromium trichloride reacts with aluminium and benzene with aluminium chloride as catalyst. The buildet complex then reacts with sodium sulphate and forms ...
Organic Chemistry Review
... Alkenes: a hydrocarbon that contains one or more carbon-carbon double bond; alkenes are unsaturated hydrocarbons. Alkynes: a hydrocarbon that contains a carbon-carbon triple bond; alkynes are unsaturated hydrocarbons. Hydrocarbon: an organic compound that contains only carbon and hydrogen. Isomer: c ...
... Alkenes: a hydrocarbon that contains one or more carbon-carbon double bond; alkenes are unsaturated hydrocarbons. Alkynes: a hydrocarbon that contains a carbon-carbon triple bond; alkynes are unsaturated hydrocarbons. Hydrocarbon: an organic compound that contains only carbon and hydrogen. Isomer: c ...
Text Questions from Corwin
... 6. What does a saturated hydrocarbon have? a single bond between each of its carbon atoms 7. Why can a carbon atom bond to as many as four other atoms? it has four valence electrons 8. What does an unsaturated hydrocarbon have? a double or triple bond between two carbon atoms 9. What characterizes a ...
... 6. What does a saturated hydrocarbon have? a single bond between each of its carbon atoms 7. Why can a carbon atom bond to as many as four other atoms? it has four valence electrons 8. What does an unsaturated hydrocarbon have? a double or triple bond between two carbon atoms 9. What characterizes a ...
4 - GEOCITIES.ws
... 3. Summarize the philosophies of vitalism and mechanism and explain how they influenced the development of organic chemistry and mainstream biological thought. a. Vitalism: a life force exists separate from physics and chemistry b. Mechanism: physical and chemical laws explain life c. Artificial org ...
... 3. Summarize the philosophies of vitalism and mechanism and explain how they influenced the development of organic chemistry and mainstream biological thought. a. Vitalism: a life force exists separate from physics and chemistry b. Mechanism: physical and chemical laws explain life c. Artificial org ...
1 - contentextra
... Aldehydes (Alkanals) A homologous series of compounds with the general formula RCHO, where the –CHO group (the aldehyde group) consists of a carbonyl group attached to a hydrogen atom. R is an alkyl or aryl group. Alkyl group A group, with the general formula CnH2n + 1, obtained by removing a hydrog ...
... Aldehydes (Alkanals) A homologous series of compounds with the general formula RCHO, where the –CHO group (the aldehyde group) consists of a carbonyl group attached to a hydrogen atom. R is an alkyl or aryl group. Alkyl group A group, with the general formula CnH2n + 1, obtained by removing a hydrog ...
unit 5 hw packet - District 196 e
... 1. Add the number of valence electrons in each atom to determine the total number of valence electrons. (For polyatomic anions, add one electron for each unit of negative charge. For polyatomic cations, subtract one electron for each unit of positive charge.) 2. Put electrons around each atom. Start ...
... 1. Add the number of valence electrons in each atom to determine the total number of valence electrons. (For polyatomic anions, add one electron for each unit of negative charge. For polyatomic cations, subtract one electron for each unit of positive charge.) 2. Put electrons around each atom. Start ...
Organic Chemistry
... an amino group (-NH2) and a carboxyl group (-COOH) Peptides—combination of amino acids where the amino group of one amino acid links to the carboxyl group of another amino acid (a peptide bond) Proteins—a peptide with more than 100 amino acids ...
... an amino group (-NH2) and a carboxyl group (-COOH) Peptides—combination of amino acids where the amino group of one amino acid links to the carboxyl group of another amino acid (a peptide bond) Proteins—a peptide with more than 100 amino acids ...
Organic Molecules and Simple Reactions
... Compounds composed entirely of carbon and hydrogen are called hydrocarbons. Hydrocarbons differ from one another by the number and arrangement of the carbon and hydrogen atoms. Other organic compounds are formed from hydrocarbons when one or more different kinds of atoms are substituted for a carbon ...
... Compounds composed entirely of carbon and hydrogen are called hydrocarbons. Hydrocarbons differ from one another by the number and arrangement of the carbon and hydrogen atoms. Other organic compounds are formed from hydrocarbons when one or more different kinds of atoms are substituted for a carbon ...
Functional Groups: Centers of Reactivity
... The general formula of a straight chain alkane is CnH2n+2. Each member of the series differs from the previous one by the addition of a –CH2-, or methylene, group. Molecules related in this manner are called homologs of each other and the series is called a homologous series. ...
... The general formula of a straight chain alkane is CnH2n+2. Each member of the series differs from the previous one by the addition of a –CH2-, or methylene, group. Molecules related in this manner are called homologs of each other and the series is called a homologous series. ...
File
... Each building represents a number in pi. Color in the number of squares on the graph paper that correspond to each digit of pi… Make a black dot to represent the decimal point… Fill in columns of squares for as many digits as will fit on one piece of paper in landscape mode. Graph ‘3’ as 3 squares – ...
... Each building represents a number in pi. Color in the number of squares on the graph paper that correspond to each digit of pi… Make a black dot to represent the decimal point… Fill in columns of squares for as many digits as will fit on one piece of paper in landscape mode. Graph ‘3’ as 3 squares – ...
Nerve activates contraction
... one of its oxygen atoms. • Phosphate groups are anions with two negative charges as two hydrogens have dissociated from the oxygen atoms. • One function of phosphate groups is to transfer energy between organic molecules. ...
... one of its oxygen atoms. • Phosphate groups are anions with two negative charges as two hydrogens have dissociated from the oxygen atoms. • One function of phosphate groups is to transfer energy between organic molecules. ...
Science 9
... magnesium, calcium, strontium, barium, and radium; all are reactive soft, low density metals. 5. ___________________ are the electrons in the outer shell of an atom, which determine its power to combine with other elements. 6. ___________________ is the regular, repeating pattern in which ions in io ...
... magnesium, calcium, strontium, barium, and radium; all are reactive soft, low density metals. 5. ___________________ are the electrons in the outer shell of an atom, which determine its power to combine with other elements. 6. ___________________ is the regular, repeating pattern in which ions in io ...
QuickStudy - Organic Chemistry Fundamentals
... Fisher-projection: diagram depicts chiral/3-D structure ...
... Fisher-projection: diagram depicts chiral/3-D structure ...
Calculating Percent Yield
... Organic reactions typically do not give 100% yields, meaning all of the starting material does not get converted to the product. The percent of starting material that is converted to product in a chemical reaction is referred to as the percent yield. The percent yield can be calculated if the follow ...
... Organic reactions typically do not give 100% yields, meaning all of the starting material does not get converted to the product. The percent of starting material that is converted to product in a chemical reaction is referred to as the percent yield. The percent yield can be calculated if the follow ...
Intro to Organic Compounds
... sodium, hafnium, or any other element. But the atomic properties of carbon do give it bonding capabilities beyond those of any other element, which in turn lead to the two obvious characteristics of organic molecules— structural complexity and chemical diversity. ...
... sodium, hafnium, or any other element. But the atomic properties of carbon do give it bonding capabilities beyond those of any other element, which in turn lead to the two obvious characteristics of organic molecules— structural complexity and chemical diversity. ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.