Unit 2 - Belle Vernon Area School District
... hydrocarbons are Aliphatic Hydrocarbons Aliphatic hydrocarbons – hydrocarbon where carbon atoms bond together in open chains ...
... hydrocarbons are Aliphatic Hydrocarbons Aliphatic hydrocarbons – hydrocarbon where carbon atoms bond together in open chains ...
Name - WordPress.com
... CH.2 THE CHEMISTRY OF LIFE 2.3 Carbon Compounds Organic Chemistry – the study of organic compounds. Organic Compounds – compounds that contain carbon. Compounds that do not usually contain carbon are inorganic compounds. Ex/ - organic compound is sugar (C, H, O) - inorganic compound is water (H, O) ...
... CH.2 THE CHEMISTRY OF LIFE 2.3 Carbon Compounds Organic Chemistry – the study of organic compounds. Organic Compounds – compounds that contain carbon. Compounds that do not usually contain carbon are inorganic compounds. Ex/ - organic compound is sugar (C, H, O) - inorganic compound is water (H, O) ...
Chapter 8 Notes - Bonding: General Concepts 8.1 Types of
... 1. Number of valence electrons on the free atom minus Number of valence electrons assigned to the atom in the molecule a. Lone pair (unshared) electrons belong completely to the atom in question b. Shared electrons are divided equally between the sharing atoms 2. The sum of the formal charges of all ...
... 1. Number of valence electrons on the free atom minus Number of valence electrons assigned to the atom in the molecule a. Lone pair (unshared) electrons belong completely to the atom in question b. Shared electrons are divided equally between the sharing atoms 2. The sum of the formal charges of all ...
Organic Chemistry
... formula but with different structural formulas • Have different chemical and physical properties ...
... formula but with different structural formulas • Have different chemical and physical properties ...
Summer Assignment 2015
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
2. Organic Families Activity
... family that each model represents and draw the expanded structural formula, the condensed structural, and the line-bond formula of the compound. If more than one family is represented you must list all of them. Most organic compounds contain a structural characteristic of the alkane family. However, ...
... family that each model represents and draw the expanded structural formula, the condensed structural, and the line-bond formula of the compound. If more than one family is represented you must list all of them. Most organic compounds contain a structural characteristic of the alkane family. However, ...
Organic Chemistry
... DNA and RNA DNA – Deoxyribonucleic Acid (Nuclear and Mitochondrial) RNA – Ribonucleic Acid (mRNA, tRNA, rRNA) The atomic number of carbon is 6, meaning 6 protons and in a stable atom 6 electrons as well. This places 4 electrons in the outer shell of a carbon atom. The presence of 4 electrons in the ...
... DNA and RNA DNA – Deoxyribonucleic Acid (Nuclear and Mitochondrial) RNA – Ribonucleic Acid (mRNA, tRNA, rRNA) The atomic number of carbon is 6, meaning 6 protons and in a stable atom 6 electrons as well. This places 4 electrons in the outer shell of a carbon atom. The presence of 4 electrons in the ...
2014MSC(ORGANIC(CHEMISTRY!
... ! The!hydrogen!that!was!attacked!will!bond!to!the!carbon!on!the!right,!due!to!Markovnikov’s! Rule!(the!rich!get!richer).! ! Although,!this!will!cause!the!double!bond!to!disappear!–!the!carbon!on!the!right!now!has!4! bonds!(the!hydrogen!replaced!one!of!its!double!bonds)!but!the!carbon!on!the!left!now ...
... ! The!hydrogen!that!was!attacked!will!bond!to!the!carbon!on!the!right,!due!to!Markovnikov’s! Rule!(the!rich!get!richer).! ! Although,!this!will!cause!the!double!bond!to!disappear!–!the!carbon!on!the!right!now!has!4! bonds!(the!hydrogen!replaced!one!of!its!double!bonds)!but!the!carbon!on!the!left!now ...
File
... Hydrolysis Reaction- a type of catabolic reaction (breaks molecules) in which water is added to break up a polymer into monomers. ...
... Hydrolysis Reaction- a type of catabolic reaction (breaks molecules) in which water is added to break up a polymer into monomers. ...
Biochemistry Worksheet
... 30. What are the 4 main elements making up proteins? How many covalent bonds does each of these elements form? 31. What are the monomers of proteins called? How many are there? Name the 4 things bonded to the center carbon of this monomer. 32. The main difference among amino acids is their _________ ...
... 30. What are the 4 main elements making up proteins? How many covalent bonds does each of these elements form? 31. What are the monomers of proteins called? How many are there? Name the 4 things bonded to the center carbon of this monomer. 32. The main difference among amino acids is their _________ ...
Chemical Bonding
... new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
... new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
Chemical Bonding
... new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
... new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
Lecture Review of Organic Chemistry and Herbicide Chemistry
... a benzene ring minus a hydrogen is termed a phenyl group and in this form can accept an appropriate substitution a replacement of the hydrogen atom with a hydroxyl (- OH) group results in the formation of phenol ...
... a benzene ring minus a hydrogen is termed a phenyl group and in this form can accept an appropriate substitution a replacement of the hydrogen atom with a hydroxyl (- OH) group results in the formation of phenol ...
Things to remember in the last hour before the exam: Level 1 Carbon
... • CO – colourless, odourless, toxic gas – stops haemoglobin in red blood cells carrying oxygen around body – so person suffocates. • C – soot; dirty & polluting. Carbon particles can cause asthma in some people and even lung cancer. Polymerisation. Monomers with C=C join together to form polymer wit ...
... • CO – colourless, odourless, toxic gas – stops haemoglobin in red blood cells carrying oxygen around body – so person suffocates. • C – soot; dirty & polluting. Carbon particles can cause asthma in some people and even lung cancer. Polymerisation. Monomers with C=C join together to form polymer wit ...
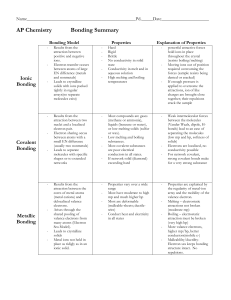
Types of Bonding Summary
... the regularity of metal-ion array and the mobility of the valence electron. ...
... the regularity of metal-ion array and the mobility of the valence electron. ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.