Formal Charge
... with four covalent bonds in any combination (four single, one double and two single, etc.) would possess 4 electrons (half of each covalent bond) and would have nv = 4, thus q = 0. An oxygen atom with three lone pairs of a electrons and one covalent bond would have n = 7 (three wholly owned pairs an ...
... with four covalent bonds in any combination (four single, one double and two single, etc.) would possess 4 electrons (half of each covalent bond) and would have nv = 4, thus q = 0. An oxygen atom with three lone pairs of a electrons and one covalent bond would have n = 7 (three wholly owned pairs an ...
Chapter 2 - Chemistry
... enzyme and its substrate (the reactant being catalyzed) Active Site: portion of the enzyme that the substrate fits into The linkage between the enzyme and the substrate causes a slight change in the enzyme’s shape which puts a strain on the substrate bonds The enzyme releases the products and ...
... enzyme and its substrate (the reactant being catalyzed) Active Site: portion of the enzyme that the substrate fits into The linkage between the enzyme and the substrate causes a slight change in the enzyme’s shape which puts a strain on the substrate bonds The enzyme releases the products and ...
Chapter 1. Electrons, Bonds and Molecular Properties
... C. Hybrid atomic orbitals and rehybridization–reconciling VSEPR and MO’s 1. sp3 orbitals a. Linear, head-on overlap of two atomic orbitals generates a σ bond b. sp3-hybridization of tetrahedral C atoms in molecules c. Orbital overlap diagrams of molecules 2. sp2 orbitals a. Linear, head-on overlap o ...
... C. Hybrid atomic orbitals and rehybridization–reconciling VSEPR and MO’s 1. sp3 orbitals a. Linear, head-on overlap of two atomic orbitals generates a σ bond b. sp3-hybridization of tetrahedral C atoms in molecules c. Orbital overlap diagrams of molecules 2. sp2 orbitals a. Linear, head-on overlap o ...
Organic Chemistry
... – The carbon chain must be numbered from the end that will give the lowest numbers for the branches Example: 2, 2, 3 – trimethyl pentane ...
... – The carbon chain must be numbered from the end that will give the lowest numbers for the branches Example: 2, 2, 3 – trimethyl pentane ...
Biochemistry Carbon Compounds
... already know that words like hydration, dehydration and dehydrated have to do with how much water you have in your body. If you look at the chemical formulas of different carbohydrates, you will see that the hydrogen (H) and the oxygen (O) are always in a 2:1 ratio, just like in water: H2O • You wil ...
... already know that words like hydration, dehydration and dehydrated have to do with how much water you have in your body. If you look at the chemical formulas of different carbohydrates, you will see that the hydrogen (H) and the oxygen (O) are always in a 2:1 ratio, just like in water: H2O • You wil ...
Topic 10 IB Chemistry Definitions
... octane rating, the less likely it is that knocking occurs. Knocking can also be reduced by antiknock agents, such as lead. Unfortunately, lead is poisonous when released into atmosphere. A unimolecular process by which a halogenoalkane undergoes nucleophilic substitution. A two-step mechanism: a rat ...
... octane rating, the less likely it is that knocking occurs. Knocking can also be reduced by antiknock agents, such as lead. Unfortunately, lead is poisonous when released into atmosphere. A unimolecular process by which a halogenoalkane undergoes nucleophilic substitution. A two-step mechanism: a rat ...
1 Atomic structure
... some have low and some high, but most have a middling energy. Reactions have a certain minimum energy requirement for a reaction to take place; this is called the activation energy. As temperature increases, the distribution moves to the right, giving a greater proportion of molecules exceeding ...
... some have low and some high, but most have a middling energy. Reactions have a certain minimum energy requirement for a reaction to take place; this is called the activation energy. As temperature increases, the distribution moves to the right, giving a greater proportion of molecules exceeding ...
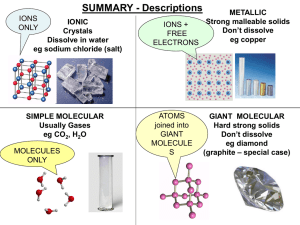
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
AP Review – Life and Chemistry Name: Date: ___B_ 1. The atomic
... charge, we know that either protons or electrons have been “lost”. If I removed protons, I’d be left with more electrons that protons which would give me a negative charge. Since there is a positive charge, I know that two negative electrons were removed (probably donated to another atom) leavin ...
... charge, we know that either protons or electrons have been “lost”. If I removed protons, I’d be left with more electrons that protons which would give me a negative charge. Since there is a positive charge, I know that two negative electrons were removed (probably donated to another atom) leavin ...
Ionic Bonding - cloudfront.net
... The 7 diatomic elements are all gases: _________________________________ ...
... The 7 diatomic elements are all gases: _________________________________ ...
AP Biology Midterm Exam Review What are atoms composed of
... Be able to explain the four levels of protein structure, what the unique features are of each level, and the bonds that are responsible for the shape of the proteins at the various levels of structure. ...
... Be able to explain the four levels of protein structure, what the unique features are of each level, and the bonds that are responsible for the shape of the proteins at the various levels of structure. ...
Ch. 8 Sections 8.1-8.3 Powerpoint
... •In ionic bonding the participating atoms are so different that one or more electrons are transferred to form oppositely charged ions, when then attract each other. •In covalent bonding (also called nonpolar covalent bonding) two identical atoms share electrons equally. •There are intermediate case ...
... •In ionic bonding the participating atoms are so different that one or more electrons are transferred to form oppositely charged ions, when then attract each other. •In covalent bonding (also called nonpolar covalent bonding) two identical atoms share electrons equally. •There are intermediate case ...
Chapter 4
... • This molecule has an OH (called a hydroxy group) attached to its backbone. • Compounds containing an OH group are called alcohols. • The hydroxy group makes the properties of ethanol very different from the properties of ethane. • Ethanol has lone pairs and polar bonds that make it reactive. ...
... • This molecule has an OH (called a hydroxy group) attached to its backbone. • Compounds containing an OH group are called alcohols. • The hydroxy group makes the properties of ethanol very different from the properties of ethane. • Ethanol has lone pairs and polar bonds that make it reactive. ...
1. Organic chemistry is the study of carbon compounds.
... Carbon chains form the skeletons of most organic molecules. The carbon skeletons may include double bonds. Hydrocarbons are organic molecules that consist of only carbon and hydrogen atoms. Isomers are compounds that have the same molecular formula but different structures and, therefore, different ...
... Carbon chains form the skeletons of most organic molecules. The carbon skeletons may include double bonds. Hydrocarbons are organic molecules that consist of only carbon and hydrogen atoms. Isomers are compounds that have the same molecular formula but different structures and, therefore, different ...
elements of chemistry unit
... C4H10 Although the two compounds above have the same molecular formula, their structural formulas are different in the way that the 4 carbons are assembled. As seen below, structure is just as essential as composition to understanding organic chemistry. C4H10 ISOMERS The two varieties of C4H10 shown ...
... C4H10 Although the two compounds above have the same molecular formula, their structural formulas are different in the way that the 4 carbons are assembled. As seen below, structure is just as essential as composition to understanding organic chemistry. C4H10 ISOMERS The two varieties of C4H10 shown ...
File
... • In Organic Chemistry, you will only be using the prefixes for one to ten. All prefixes have the ending of “yl” but is removed for specific families/groups. Details later. • 1 – meth 6 - hex • 2 – eth 7 - hept • 3 – prop 8 - oct • 4 – but 9 - non • 5 – pent 10 - dec ...
... • In Organic Chemistry, you will only be using the prefixes for one to ten. All prefixes have the ending of “yl” but is removed for specific families/groups. Details later. • 1 – meth 6 - hex • 2 – eth 7 - hept • 3 – prop 8 - oct • 4 – but 9 - non • 5 – pent 10 - dec ...
ORDANOCHROMIUM CHEMISTRY SUPPORTED BY -DIIMINE LIGANDS
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.