Elements, Compounds, Mixtures wrksht
... Elements, Compounds, Mixtures & Atoms Classify each of the pictures below by placing the correct label ...
... Elements, Compounds, Mixtures & Atoms Classify each of the pictures below by placing the correct label ...
1b. Loss of N
... be transported out more slowly; so heart muscle is exposed to Ca2+ for a longer period of time. As a result, the heart contracts more forcefully (“inotropic effect”) It also increases cholinergic stimulation to the heart, which slows it down (steadier, stronger heartbeat with more rest between beats ...
... be transported out more slowly; so heart muscle is exposed to Ca2+ for a longer period of time. As a result, the heart contracts more forcefully (“inotropic effect”) It also increases cholinergic stimulation to the heart, which slows it down (steadier, stronger heartbeat with more rest between beats ...
organic chem ppt notes
... A compound is chiral when it cannot be superimposed on its mirror image. The pair of mirror imaged non-superimposable compounds are known as enantiomers. Even though very similar still, different enantiomers of the same chiral drug can have very different pharmological effects, mainly because the pr ...
... A compound is chiral when it cannot be superimposed on its mirror image. The pair of mirror imaged non-superimposable compounds are known as enantiomers. Even though very similar still, different enantiomers of the same chiral drug can have very different pharmological effects, mainly because the pr ...
Mass Spec - Fragmentation
... 1. The relative height of the M+ peak is greatest for straightchain molecules and decreases as the branching increases. 2. The relative height of the M+ peak decreases with chain length for a homologous series. 3. Cleavage is favoured at alkyl-substituted carbons, with the probability of cleavage in ...
... 1. The relative height of the M+ peak is greatest for straightchain molecules and decreases as the branching increases. 2. The relative height of the M+ peak decreases with chain length for a homologous series. 3. Cleavage is favoured at alkyl-substituted carbons, with the probability of cleavage in ...
PowerPoint Presentation - Slide 1 - University of Evansville Faculty
... on its carbon skeleton, but also on certain groups of atoms that are covalently linked to the skeleton. These groups of atoms are called functional groups, the name reflecting the fact that these parts of the organic molecules usually are involved in chemical reactions. See Table 4.1 in Campbell et ...
... on its carbon skeleton, but also on certain groups of atoms that are covalently linked to the skeleton. These groups of atoms are called functional groups, the name reflecting the fact that these parts of the organic molecules usually are involved in chemical reactions. See Table 4.1 in Campbell et ...
Word - chemmybear.com
... Atoms tend to lose, gain, or ___________ electrons to complete their valence shells. When a chlorine atom gains an electron, it fills its valence shell forming a negative chloride________. Whenever ionic solids are formed, __________ is involved. An ionic material is composed of positive ions bonded ...
... Atoms tend to lose, gain, or ___________ electrons to complete their valence shells. When a chlorine atom gains an electron, it fills its valence shell forming a negative chloride________. Whenever ionic solids are formed, __________ is involved. An ionic material is composed of positive ions bonded ...
lec-2- 211(ES +Add)
... •Which is an empirical rule based on Markovnikov's experimental observations on the addition of hydrogen halides to alkenes. •The rule states that : "when an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater ...
... •Which is an empirical rule based on Markovnikov's experimental observations on the addition of hydrogen halides to alkenes. •The rule states that : "when an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater ...
Chemistry-Chapter 2 Lecture Notes Page
... * Hydrogen * Oxygen * Carbon * Nitrogen - Each element composed of similar atoms ...
... * Hydrogen * Oxygen * Carbon * Nitrogen - Each element composed of similar atoms ...
Chapter 2
... partial charges The atoms are not ions, the partial charges result from the atoms being polar covalently bonded to some other atom. weak bonds, but very important in living systems ...
... partial charges The atoms are not ions, the partial charges result from the atoms being polar covalently bonded to some other atom. weak bonds, but very important in living systems ...
Lecture 2
... Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized and partly because their metabolism produces substances that are even more toxic. Methanol (wood alcohol), for instance, is oxidized to formaldehyde and then to the poisonous formic a ...
... Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized and partly because their metabolism produces substances that are even more toxic. Methanol (wood alcohol), for instance, is oxidized to formaldehyde and then to the poisonous formic a ...
Functional Groups
... Rule #1: Identify the longest chain of carbon atoms a) The longest chain of carbon atoms gives the stem/root of the name as shown in the table below: # of C-atoms in Stem in longest chain IUPAC name ...
... Rule #1: Identify the longest chain of carbon atoms a) The longest chain of carbon atoms gives the stem/root of the name as shown in the table below: # of C-atoms in Stem in longest chain IUPAC name ...
The characteristic reaction of aromatic rings
... Reactions of the Side Chain of Alkylbenzenes t Benzylic Radicals and Cations l When toluene undergoes hydrogen abstraction from its methyl group it produces a benzyl radical è A benzylic radical is a radical in which the carbon bearing the unpaired electron is directly bonded to an aromatic ring ...
... Reactions of the Side Chain of Alkylbenzenes t Benzylic Radicals and Cations l When toluene undergoes hydrogen abstraction from its methyl group it produces a benzyl radical è A benzylic radical is a radical in which the carbon bearing the unpaired electron is directly bonded to an aromatic ring ...
3.2-3.3 GN
... Dehydration synthesis removes the _____________ from the glycerol and an _____ from the fatty acid to form a ____________________ bond. ...
... Dehydration synthesis removes the _____________ from the glycerol and an _____ from the fatty acid to form a ____________________ bond. ...
Organic Chemistry - City University of New York
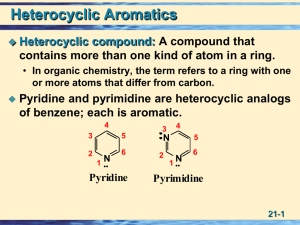
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
Review of Definitions
... 11. Hydrophilic Molecules. Molecules which can form hydrogen bonds with water and hence, are miscible with water, i.e."water-loving," (e.g. sugars, alcohols, soluble proteins, DNA, poly(ethylene oxide)). 12. Bond Rupture Strength. Binding energy needed to break a bond. 13.Ionization. Any process by ...
... 11. Hydrophilic Molecules. Molecules which can form hydrogen bonds with water and hence, are miscible with water, i.e."water-loving," (e.g. sugars, alcohols, soluble proteins, DNA, poly(ethylene oxide)). 12. Bond Rupture Strength. Binding energy needed to break a bond. 13.Ionization. Any process by ...
Ch04 Organic Chem 9e
... Pick up a copy of the “Functional Groups” chart on the back counter. Use pages 64-65 to fill it out. ...
... Pick up a copy of the “Functional Groups” chart on the back counter. Use pages 64-65 to fill it out. ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.