* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gr - loyolascience2
Survey
Document related concepts
Transcript
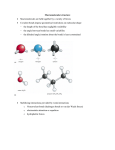
I. ORGANIC CHEMISTRY: Introduction to Naming, Drawing, Structure, and Properties The Organization of Hydrocarbons 1. Read Section 1.2 (p. 11 – 23). 2. Additional Notes Organization of Hydrocarbons Hydrocarbons (carbon and hydrogen atoms only; backbone of all organic compounds) Aliphatic Compounds Aromatic Compounds (non-aromatic) (contain alternating double bonds) Open Chain Cyclic Open Chain (aka acyclic) (acyclic, ex.tail of Vitamin.A) Straight Chain Cyclic (multiples of benzene) Branched (unbranched) Saturated (single bonds only in parent chain) Unsaturated (at least one multiple bond in parent chain) Substituted Organic Family Linkages (side groups off parent chain ex. -Br, -Cl, -OH,-NH2) (cpd named after one prominent side group ex. alcohols, ketones) (parent chain is interrupted by a non-carbon atom ex. ethers, esters) Drawing Organic Compounds The method for drawing organic compounds used in the textbook is a hybrid method of two types of chemical formulas: the structural formula and the condensed structural formula. While this method is commonly used as a short cut by experienced chemists, it is important that you first learn to draw organic compounds using structural formulas only to reveal all atoms and bonds. You will learn how to draw condensed structural formulas as a separate skill. Go to the Learning Tip on p. 15. The compound here is represented in the following ways: (a) a hybrid of a structural formula and a condensed structural formula; (b) a spacefill diagram; (c) a wireframe diagram (the most convenient method for drawing large biochemicals; and (d) a ball and stick diagram (called a bond and angle diagram in your text), which most closely represents a structural formula as you are expected to draw them. The only shortcuts you are allowed are (1) leaving out the hydrogens attached to carbons (showing sticks only), and (2) showing hydroxyl as OH and not O–H. Ex. 1-ethylcyclohexane Structural Formula “Hybrid” Formula Condensed Struct. Formula Molecular Formula Functional Groups: Read Section 1.1 (p. 8 - 10) Additional Notes: Functional Groups vs. Linkages A functional group is a site of chemical reactivity within an organic molecule. It can be a side group or a feature of the parent chain, such as a multiple bond. Chemical reactivity depends upon atoms of differing electronegativities. An alkane contains no unsaturation or polar groups; therefore, it contains no functional group. If a molecule is unsaturated or contains some element besides carbon and hydrogen, the molecule contains at least one functional group. Ex. CH3CH2CH2CH3 CH3CH2CH=CH2 CH3CH2CH2CH2OH no functional group functional groups Often initially classified as functional groups, linkages not only offer reactivity to the molecule, but also introduce a non-carbon atom to the parent chain. Longer molecules are often made from smaller ones through linkages. Ex. Esters CH3CH2C-O2-CH2CH3 ethylpropanoate Ethers CH3CH2-O-CH2CH3 ethoxyethane (diethyl ether) 2 Comparison Chart of the Characteristics of C-C Single, Double, and Triple Bonds Characteristic Rel. Bond Length C-C Single Bond longest C-C Double Bond Shorter C-C Triple Bond shortest Overall Bond Strength weakest Stronger strongest # sigma bonds # pi bonds stability of bond structure 1 1 1 0 high 1 Lower 2 lowest reactivity low (highly unreactive) Higher highest Reasoning attraction between carbon bonds increases as number of shared pairs increases as the number of bonds between atoms increases, the energy required to separate the atoms increases as well a sigma bond forms before any pi bonds can form a pi bond is more unstable than a sigma bond because the electron pair is shared by the sideways overlap of p orbitals; these shared electrons are not as tightly held by either nucleus as they would be in the end-on-end overlap of sigma bonds very little energy is required to remove the electrons from a pi bond because the electrons are further away from the nuclei of each atom and are not tightly held by each nucleus “Like Dissolves Like” Why is it true that polar substances dissolve in polar solvents and non-polar substances dissolve in non-polar solvents? There are two important tendencies to consider. First, a substance will dissolve at all if the attraction between solute and solvent particles is stronger than the attraction between the solute particles. This makes sense with polar solvents dissolving polar compounds. Highly soluble compounds are easily hydrated by water because water particles are very polar, and fluid, and are able to pull solute particles towards them and surround them relatively easily. In fact, this process usually releases energy. But how does this explain the circumstance when non-polar solvents dissolve nonpolar substances? The forces of attraction among any of these particles are too weak for the explanation of greater attraction to be adequate. The key here is twofold. First, it is important to note that polar solvents have relatively no effect on non-polar solutes by way of attraction. Having said that, it is now important to consider the second tendency: all particles seek greatest randomness. It is not that non-polar solvents have a stronger attraction for non-polar solutes, but rather that there is no specific attraction at all, which allows solute particles to diffuse readily according to the 2 nd law of thermodynamics – all particles seek greatest randomness. It is the lack of attraction among particles, then, that actually allows non-polar solute particles to mix readily with non-polar solvent particles. Like dissolves like! 3 The Effect of the Hydroxyl Group, -C-O-H, vs. the Carbonyl Group, -C=O, on the Boiling Point of an Organic Compound The hydroxyl group, -C-O-H, the principal functional group of the alcohol organic family, can form hydrogen bonds with other alcohol molecules. This is the strongest intermolecular force possible between particles. Carbonyl groups, -C=O, the principal functional group of aldehydes and ketones, are strongly polar, but can still only form dipoledipole forces of attraction between neighbouring molecules. These are not as strong as hydrogen bonds. Therefore, alcohols tend to have higher boiling points than aldehydes and ketones of comparable length. Similarly, alcohols can be more soluble in water than aldehydes and ketones of comparable length. Practice: - Section 1.1 Q’s: #1, 2 (redraw as structural formulas first) (p. 10) - #1-3, 5-8 (pp. 15-21) (use the following nomenclature rules) 4 Organic Chemistry Nomenclature Rules SUMMARY 1. Find the longest hydrocarbon chain in the molecule and use the appropriate prefix to designate the number of carbons in this parent chain (ex. meth-, eth-, prop-, etc.). If an aliphatic ring system is present, it must be chosen as the parent chain. 2. Choose the appropriate ending to indicate the classification of the molecule (ex. alkane, -ane; alkene, ene; alkyne, -yne; alcohol, -ol; aldehyde, -al; ketone, -one; carboxylic acid, -oic acid; ester, -oate; etc.). 3. Hydrocarbon branches are named according to their size (using the appropriate prefix) and end in –yl (ex. 2 carbon hydrocarbon branch, ethyl). You must indicate their location on the parent chain by numbering them according to the carbons to which they are attached. Other functional groups are added and numbered in the same fashion. 4. For molecules containing multiple bonds, the main chain must contain the double or triple bond; the numbering is assigned so that the multiple bond has the lower number. 5. Numbering: a. numbering the positions of side groups along a parent chain must start from the end that results in a set of position numbers with the smallest total. Ex. 3,4-dichloromethylpentane is identical to 2,3-dichloro-methylpentane but the former adds to 7 and the latter adds to 5; therefore, the latter is correct and the former is incorrect. b. when two or more of the same side group are present in the same molecule, you must add a prefix to the group name that indicates how many there are (ex. di, tri, tetra, etc.). Ex. c. 2,2,3-trichlorohexane and not 2,2,3-chlorohexane for two different side groups that have the same positions in a molecule, the order is determined by decreasing chemical reactivity or complexity. If these are similar or not known, then a numerical order will suffice. Ex. 2-nitro-4-methylpentane and not 2-methyl-4-nitropentane d. for aliphatic ring systems, the prefix “cyclo” must precede the side or parent chain name. 6. Written format: Commas between position numbers, hyphens between numbers and words, and no spaces between words (i.e. last listed side group and parent name) Ex. 2,3-dichloro-2-ethyl-3-methylheptane Note: If you were to draw the above compound, you would discover that its name should be changed. What should it be changed to? 5 6 How Aromatics are Different from Ordinary Unsaturates The situation of alternating double bonds throughout a cyclic or acyclic organic compound is unique in that a pi bond forms between each successive carbon throughout the molecule (or section of the molecule, as in the case of Vitamin A). This allows p-orbital electrons to become delocalized above and below the plane of the molecule to form “pi-clouds” (the plane of the molecule is formed by sigma bonds). Multiple Names Figure 7 (p. 19) shows the condensed structural formula of the molecule methylbenzene, which can also be named phenylmethane – both names being acceptable by IUPAC. Its common name is toluene. On a separate page, draw both the structural and condensed structural formulas for this molecule, and list the names below each one. Copy the captions from the text below each one as well. Complete the following: 1. Commit Table 2 (p.12) to memory! 2. Learn the common, non-systematic prefixes used for the skeletal isomers in Figure 5 (P. 13) (n, iso, s, t); write out the corresponding IUPAC names for each isomer. 3. Answer: #1 (b-d) and 2 (p.16) #3 – 6 (p. 18-19) 4. Draw Fig. 8 (p. 19) into the space below. Write corresponding names below the systematic names using the classical system described in the Learning Tip on p. 20. 5. Answer: #7 (redraw each molecule using proper structural formulas), #8 (p. 21) #1 (not a typo in (6), it should be “iso”), #2 (draw a structural formula first, then answer), #3, 4 (p. 22-23) 7 8 9 10 11 12 13 14 15 16 17 18 19