Chemistry: Matter and Change
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
HOW DOES THE SUN GENERATE ENERGY? - IDC
... Matter is still in the form of plasma (the vast majority of hydrogen ions), but begins to have a behavior similar to an ocean. Convection processes occur where spin columns will generate large amounts of heat that carry hot materials to the photosphere of the Sun and other ionized atoms returning le ...
... Matter is still in the form of plasma (the vast majority of hydrogen ions), but begins to have a behavior similar to an ocean. Convection processes occur where spin columns will generate large amounts of heat that carry hot materials to the photosphere of the Sun and other ionized atoms returning le ...
Physics 847: Problem Set 7
... (b). Calculate the spin wave spectrum for this Hamiltonian, following the approach used in class. Show, in particular, that the spectrum has a gap, i. e., the lowest spin wave excitation has an energy of ∆ 6= 0, and find ∆. What is the temperature dependence of the spin wave specific heat in this ca ...
... (b). Calculate the spin wave spectrum for this Hamiltonian, following the approach used in class. Show, in particular, that the spectrum has a gap, i. e., the lowest spin wave excitation has an energy of ∆ 6= 0, and find ∆. What is the temperature dependence of the spin wave specific heat in this ca ...
Chapter 3
... The Chemistry of Life • Matter- anything that has mass and takes up space – Solid: any substance that has a definite shape and a definite volume – Liquid: has a definite volume but no definite shape – Gas: has no definite volume or shape *Changing states requires adding or removing energy ...
... The Chemistry of Life • Matter- anything that has mass and takes up space – Solid: any substance that has a definite shape and a definite volume – Liquid: has a definite volume but no definite shape – Gas: has no definite volume or shape *Changing states requires adding or removing energy ...
Chapter 6 Notes - Discount Flies
... Coeffecient = a whole number written in front of a substance which indicates the number of molecules that react. Tricks for balancing: 1. Write correct formulas for reactants and products first. Don’t ever change the formula of a substance once it is written correctly. 2. Balance O and H last. ...
... Coeffecient = a whole number written in front of a substance which indicates the number of molecules that react. Tricks for balancing: 1. Write correct formulas for reactants and products first. Don’t ever change the formula of a substance once it is written correctly. 2. Balance O and H last. ...
Ch 12: Electromagnetic Waves
... can behave as a particle, called a photon, whose energy depends on the frequency of the waves. ...
... can behave as a particle, called a photon, whose energy depends on the frequency of the waves. ...
(2) Gph 321- MECHANISM OF ELECTRICAL
... greater than a few kilometers, where pore spaces have been closed by high pressure, thus studies involving conductivity of the deep crust and mantle require other mechanisms than ion flow through connate water. c) Polarization of ions or sometimes electrons under the influence of an electrical field ...
... greater than a few kilometers, where pore spaces have been closed by high pressure, thus studies involving conductivity of the deep crust and mantle require other mechanisms than ion flow through connate water. c) Polarization of ions or sometimes electrons under the influence of an electrical field ...
Chemistry: Matter and Change
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
Fall Final Rev 2014
... 20 a. Covalent bonding: nonmetals share valence electrons w other nonmetals so each atom is surrounded by 8 (or 2, for H). Atoms stay w their valence e– to form molecules. Molecule has no charge, so it does not conduct electricity in any state. Ionic bonding: metals transfer e– to nonmetals; metals ...
... 20 a. Covalent bonding: nonmetals share valence electrons w other nonmetals so each atom is surrounded by 8 (or 2, for H). Atoms stay w their valence e– to form molecules. Molecule has no charge, so it does not conduct electricity in any state. Ionic bonding: metals transfer e– to nonmetals; metals ...
PRACTICE EXAM for FALL 2013 FINAL EXAM (Unit 6 + review) 1
... 20 a. Covalent bonding: nonmetals share valence electrons w other nonmetals so each atom is surrounded by 8 (or 2, for H). Atoms stay w their valence e– to form molecules. Molecule has no charge, so it does not conduct electricity in any state. Ionic bonding: metals transfer e– to nonmetals; metals ...
... 20 a. Covalent bonding: nonmetals share valence electrons w other nonmetals so each atom is surrounded by 8 (or 2, for H). Atoms stay w their valence e– to form molecules. Molecule has no charge, so it does not conduct electricity in any state. Ionic bonding: metals transfer e– to nonmetals; metals ...
Chapter 2
... • If the number of prey decreases in an ecosystem, then some predators starve, and ...
... • If the number of prey decreases in an ecosystem, then some predators starve, and ...
Chapter 6 CHEM 121
... IDEAL GASES vs. REAL GASES • No ideal gases actually exist. • If they did exist, they would behave exactly as predicted by the gas laws at all temperatures and pressures. • Real gases deviate from the behavior predicted by the gas laws, but under normally encountered temperatures and pressures, the ...
... IDEAL GASES vs. REAL GASES • No ideal gases actually exist. • If they did exist, they would behave exactly as predicted by the gas laws at all temperatures and pressures. • Real gases deviate from the behavior predicted by the gas laws, but under normally encountered temperatures and pressures, the ...
Liquid_Nitrogen_Session2
... Tuesday the 17 of 2007 our group worked with liquid nitrogen (LN2) and observed the different outcomes depended on the cup holding the LN2. We used three different types of cups (plastic, Styrofoam, and vacuum) to hold the nitrogen, which were in different environments such as ice baths, with a lid ...
... Tuesday the 17 of 2007 our group worked with liquid nitrogen (LN2) and observed the different outcomes depended on the cup holding the LN2. We used three different types of cups (plastic, Styrofoam, and vacuum) to hold the nitrogen, which were in different environments such as ice baths, with a lid ...
Plasma ion-assisted deposition coating system
... crucible. Intense local heating melts and vaporizes some of the coating material in the center of the crucible without causing undue heating of the crucible itself. For particularly involatile materials, the electron gun can be focused to intensify its effects. Careful control of the temperature and ...
... crucible. Intense local heating melts and vaporizes some of the coating material in the center of the crucible without causing undue heating of the crucible itself. For particularly involatile materials, the electron gun can be focused to intensify its effects. Careful control of the temperature and ...
Plasma
... • The ion beam passes the Magnetic sector for mass separation, and the Electrostatic sector (ESA) for energy separation and energy focusing. • Ion detection behind the Exit Slit is realized by a Conversion Dynode and an ‘off-axis’ Secondary Electron Multiplier (SEM). ...
... • The ion beam passes the Magnetic sector for mass separation, and the Electrostatic sector (ESA) for energy separation and energy focusing. • Ion detection behind the Exit Slit is realized by a Conversion Dynode and an ‘off-axis’ Secondary Electron Multiplier (SEM). ...
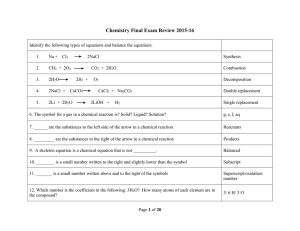
Type Of Chemical Reaction
... 13. Which law states that the number of each type of atom in the reactants must equal the number of atoms of each type in the product? ...
... 13. Which law states that the number of each type of atom in the reactants must equal the number of atoms of each type in the product? ...
1 - contentextra
... radiation and moves from a lower energy level to higher energy level. Acceleration The stage in a mass spectrometer when the positive ions are attracted to negatively charged plates. They are accelerated by an electric field. Adsorption The process, usually temporarily, when gases, liquids or solute ...
... radiation and moves from a lower energy level to higher energy level. Acceleration The stage in a mass spectrometer when the positive ions are attracted to negatively charged plates. They are accelerated by an electric field. Adsorption The process, usually temporarily, when gases, liquids or solute ...
Multiple choice revision quiz
... D. The number of neutrons can be worked out by subtracting the atomic number from the mass number 7. Which statement about the Halogens is incorrect? A. They exist as diatomic molecules like Cl2 B. They have 7 electrons in the outer shell 8. Which statement about sodium is incorrect? A. It burns wit ...
... D. The number of neutrons can be worked out by subtracting the atomic number from the mass number 7. Which statement about the Halogens is incorrect? A. They exist as diatomic molecules like Cl2 B. They have 7 electrons in the outer shell 8. Which statement about sodium is incorrect? A. It burns wit ...
Unit 1B1 - Uddingston Grammar School
... Atoms P and Q have the same number of protons Atoms Q and R have the same number of electrons Atoms P and S have the same number of neutrons Atoms R and S are isotopes of each other Atoms S and T have different chemical properties. ...
... Atoms P and Q have the same number of protons Atoms Q and R have the same number of electrons Atoms P and S have the same number of neutrons Atoms R and S are isotopes of each other Atoms S and T have different chemical properties. ...
Ch.1-Matter and Change
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
... In the liquid state, matter has a definite volume, but an indefinite shape. In the gaseous state, matter has neither definite volume nor definite shape. Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
Nemcova abstract- ICPIG.rtf - Queen`s University Belfast
... possibility of application in nanoscience [1-4]. There are many different approaches to such plasma production, the main difference being in the electrode configuration. However in virtually all cases there are highly reactive species generated in the liquid particularly OH, O3, O oxygen radicals an ...
... possibility of application in nanoscience [1-4]. There are many different approaches to such plasma production, the main difference being in the electrode configuration. However in virtually all cases there are highly reactive species generated in the liquid particularly OH, O3, O oxygen radicals an ...
Chemistry Study Guide
... Mixture Suspension Chemical property A liquid changes into a gas through evaporation When ingredients are evenly mixed a solution is formed. When iron turns to rust it is an example of a chemical property. When ingredients can easily be seen such as the ingredients in salad dressing it is called a s ...
... Mixture Suspension Chemical property A liquid changes into a gas through evaporation When ingredients are evenly mixed a solution is formed. When iron turns to rust it is an example of a chemical property. When ingredients can easily be seen such as the ingredients in salad dressing it is called a s ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).