Course 3: Pressure – Volume – Temperature Relationship of Pure
... Ideal gas equation of state is a model equation applicable to all gases to understand their P-V-T behavior and the energy requirements of processes within small margins of error Consider an engine piston full of ideal gas Total energy of the ideal gas can only be changed through transfer of energy ...
... Ideal gas equation of state is a model equation applicable to all gases to understand their P-V-T behavior and the energy requirements of processes within small margins of error Consider an engine piston full of ideal gas Total energy of the ideal gas can only be changed through transfer of energy ...
10 08 Fall economisers on screw compressors The refrigerant
... This Activity received funding from the Department of Industry as part of the Energy Efficiency Information Grants Program. The views expressed herein are not necessarily the views of the Commonwealth of Australia, and the Commonwealth does not accept responsibility for any information or advice con ...
... This Activity received funding from the Department of Industry as part of the Energy Efficiency Information Grants Program. The views expressed herein are not necessarily the views of the Commonwealth of Australia, and the Commonwealth does not accept responsibility for any information or advice con ...
Test 2
... solids, tell how they are different from each other, and give an example of each. Molecular - have complete molecules at lattice points Ionic - Have ins at lattice points Atomic- Have atoms at lattice points 6. (5 points) What kind of bonding is used to hold a metal lattice together, and how is this ...
... solids, tell how they are different from each other, and give an example of each. Molecular - have complete molecules at lattice points Ionic - Have ins at lattice points Atomic- Have atoms at lattice points 6. (5 points) What kind of bonding is used to hold a metal lattice together, and how is this ...
Pharmaceutical Particles
... When a sufficiently high voltage is applied to a liquid droplet, the body of the liquid becomes charged, and electrostatic repulsion counteracts the surface tension and the droplet is stretched; at a critical point a stream of liquid erupts from the surface. This point of eruption is known as the Ta ...
... When a sufficiently high voltage is applied to a liquid droplet, the body of the liquid becomes charged, and electrostatic repulsion counteracts the surface tension and the droplet is stretched; at a critical point a stream of liquid erupts from the surface. This point of eruption is known as the Ta ...
Ang. bindningstyper och elektronegativitet
... electronegativity [difference] of [atoms in] these bonds is 0.3 to 1.7. Ionic bonding is a type of electrostatic interaction between atoms which have a large electronegativity difference. There is no precise value that distinguishes ionic from covalent bonding, but a difference of electronegativity ...
... electronegativity [difference] of [atoms in] these bonds is 0.3 to 1.7. Ionic bonding is a type of electrostatic interaction between atoms which have a large electronegativity difference. There is no precise value that distinguishes ionic from covalent bonding, but a difference of electronegativity ...
LIQUIDS
... More air needs to be submerged to balance extra weight Boat still floats, but now a little lower ...
... More air needs to be submerged to balance extra weight Boat still floats, but now a little lower ...
Lecture 13
... a changing tilt with time, then over millions of years life would have to adjust. This is long enough that I don’t think it would be a disqualifier. The orbit is potentially another story. Earth’s orbit, like the orbit of the other major planets, is close to circular. Now, any stellar planetary syst ...
... a changing tilt with time, then over millions of years life would have to adjust. This is long enough that I don’t think it would be a disqualifier. The orbit is potentially another story. Earth’s orbit, like the orbit of the other major planets, is close to circular. Now, any stellar planetary syst ...
Why do scientists believe that?
... are produced when energetic electrons are stopped in matter. (Inverse photo-electric effect.) The Compton effect proves that photons act like particles with energy and momentum. When they scatter from electrons in matter they lose energy in a particular way, related to the angle of scattering. For r ...
... are produced when energetic electrons are stopped in matter. (Inverse photo-electric effect.) The Compton effect proves that photons act like particles with energy and momentum. When they scatter from electrons in matter they lose energy in a particular way, related to the angle of scattering. For r ...
Chapter 1 and Sections 3.1-3.3
... Chemistry is the study of the composition of matter (substances) and the way in which they interact physically and chemically over time. Energy is involved in every change/transformation of matter. Chemists work to characterize or analyze the composition, structure and properties of matter and the c ...
... Chemistry is the study of the composition of matter (substances) and the way in which they interact physically and chemically over time. Energy is involved in every change/transformation of matter. Chemists work to characterize or analyze the composition, structure and properties of matter and the c ...
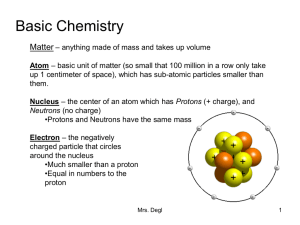
Basic Chemistry
... Isotopes – Atoms of the same element can have a different number of neutrons. Some Carbon atoms have 6 neutrons and some have 7/ 8. •Some isotopes can be radioactive (unstable nuclei that breaks down at a constant rate over time). Compound – when two or more elements are chemically combined •Bonds ...
... Isotopes – Atoms of the same element can have a different number of neutrons. Some Carbon atoms have 6 neutrons and some have 7/ 8. •Some isotopes can be radioactive (unstable nuclei that breaks down at a constant rate over time). Compound – when two or more elements are chemically combined •Bonds ...
Summer Assignment 2015
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
How do we maximize performance from our PCs?
... • Electronic thermodynamics show that CPUs run more efficiently under cold temperatures • Maximizing speeds and performance requires more voltage supplied to the CPU • More voltage = More heat • With extremely low temperatures, heat inefficiency is negated! • How do we get these freezing temperature ...
... • Electronic thermodynamics show that CPUs run more efficiently under cold temperatures • Maximizing speeds and performance requires more voltage supplied to the CPU • More voltage = More heat • With extremely low temperatures, heat inefficiency is negated! • How do we get these freezing temperature ...
Ch 6 Jeopardy Review
... In these bonds valence electrons are able to freely move between a cation lattice. ...
... In these bonds valence electrons are able to freely move between a cation lattice. ...
P340_2011_week10
... We will discuss this in more detail toward the end of the semester, but it is possible to slow-down (cool) atoms by passing them through a region with counter-oriented laser beams tuned to just below an optical transition [“optical molassses”; so that atoms moving toward the laser will see photons D ...
... We will discuss this in more detail toward the end of the semester, but it is possible to slow-down (cool) atoms by passing them through a region with counter-oriented laser beams tuned to just below an optical transition [“optical molassses”; so that atoms moving toward the laser will see photons D ...
Resistance of constantan wire
... As you may know the current in a wire is due to the motion of free electrons within the wire. These electrons are not bound to any particular atom but are free to 'wander' through the body of the material. The more free electrons per unit volume the greater the current for a given voltage difference ...
... As you may know the current in a wire is due to the motion of free electrons within the wire. These electrons are not bound to any particular atom but are free to 'wander' through the body of the material. The more free electrons per unit volume the greater the current for a given voltage difference ...
Chemistry Game - Ceres Unified School District
... helium lithium Boron Carbon Oxygen Fluorine Neon and others ...
... helium lithium Boron Carbon Oxygen Fluorine Neon and others ...
Covalent Bonds
... Molecules can be polar if electrons are unevenly shared within the molecule - The more electronegative atom will be partially negative - The less electronegative atom will be partially positive ...
... Molecules can be polar if electrons are unevenly shared within the molecule - The more electronegative atom will be partially negative - The less electronegative atom will be partially positive ...
AQA Core Science Final Test - Atoms and Chemical equations
... of the scientific response. Teachers should and apply a ‘best-fit’ approach to the marking. ...
... of the scientific response. Teachers should and apply a ‘best-fit’ approach to the marking. ...
File
... other! Hydrogen bonds hold water molecules together. When you see a water drop, it looks like a ball because the molecules stick together! Cohesion is the attraction of particles of the same substance. ...
... other! Hydrogen bonds hold water molecules together. When you see a water drop, it looks like a ball because the molecules stick together! Cohesion is the attraction of particles of the same substance. ...
Chapter 9: Chemical Quantities
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
1 Dark Matter as a consequence of electric charge non
... charged and neutral particles. In accordance with what is known at present about inflation charged particles disappeared during inflation. This observation due initially to Guth [2] has remained unchallenged by the subsequent formulations of inflation. From the point of view of the early Universe th ...
... charged and neutral particles. In accordance with what is known at present about inflation charged particles disappeared during inflation. This observation due initially to Guth [2] has remained unchallenged by the subsequent formulations of inflation. From the point of view of the early Universe th ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).