Honors Chemistry Final Review
... When given the formula, and writing the name for ionic compounds, remember to place the names in the same order as they appear in the formula. Remember, that if a transition metal, tin or lead appears, you will need a Roman Numeral. The Roman Numeral indicates the charge of the metal ion, and while ...
... When given the formula, and writing the name for ionic compounds, remember to place the names in the same order as they appear in the formula. Remember, that if a transition metal, tin or lead appears, you will need a Roman Numeral. The Roman Numeral indicates the charge of the metal ion, and while ...
Name ………………………………………………… Unit 7: States of
... (1) Propanone has a higher vapor pressure and stronger intermolecular forces than water. (2) Propanone has a higher vapor pressure and weaker intermolecular forces than water. (3) Propanone has a lower vapor pressure and stronger intermolecular forces than water. (4) Propanone has a lower vapor pres ...
... (1) Propanone has a higher vapor pressure and stronger intermolecular forces than water. (2) Propanone has a higher vapor pressure and weaker intermolecular forces than water. (3) Propanone has a lower vapor pressure and stronger intermolecular forces than water. (4) Propanone has a lower vapor pres ...
Thermodynamic Symbols and Constants
... H is the enthalpy (= E + PV); (J/mol) G is the Gibbs energy (= H – TS); (J/mol) Cp is the heat capacity at constant temperature; (J/mol K) The superscript o denotes the value given is for the standard reference state. For gases this is the ideal gas state at 1 atm. For example Hv refers to the enth ...
... H is the enthalpy (= E + PV); (J/mol) G is the Gibbs energy (= H – TS); (J/mol) Cp is the heat capacity at constant temperature; (J/mol K) The superscript o denotes the value given is for the standard reference state. For gases this is the ideal gas state at 1 atm. For example Hv refers to the enth ...
Physical Science - Edgemead High School
... charged objects have an excess of electrons Describe how objects (insulators) can be charged by contact (or rubbing) tribo-electric charging. Tribo-electric charging: A type of contact electrification in which certain materials become electrically charged after they come into contact with differen ...
... charged objects have an excess of electrons Describe how objects (insulators) can be charged by contact (or rubbing) tribo-electric charging. Tribo-electric charging: A type of contact electrification in which certain materials become electrically charged after they come into contact with differen ...
Ch 1 notes
... 1. General: Do all calculations, keeping track of the significant figures as you go, but do not round until the end 2. Note: the basis for this method is that you cannot have more than one estimated digit. 3. Addition/Subtraction rule: ...
... 1. General: Do all calculations, keeping track of the significant figures as you go, but do not round until the end 2. Note: the basis for this method is that you cannot have more than one estimated digit. 3. Addition/Subtraction rule: ...
Physics Qualifier Part I—Spring 2010 7-Minute Questions α
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
GRE-thermo
... 13. A large isolated system of N weakly interacting particles is in thermal equilibrium. Each particle has only 3 possible nondegenerate states of energies 0, , and 3. When the system is at an absolute temperature T >> /k, where k is Boltzmann's constant, the average energy of each particle is ...
... 13. A large isolated system of N weakly interacting particles is in thermal equilibrium. Each particle has only 3 possible nondegenerate states of energies 0, , and 3. When the system is at an absolute temperature T >> /k, where k is Boltzmann's constant, the average energy of each particle is ...
Lecture #21 04/14/05
... an electron moving in a loop around a nucleus, then we have a current loop. nuclei ...
... an electron moving in a loop around a nucleus, then we have a current loop. nuclei ...
Export To Word
... change in motion. When objects travel at speeds comparable to the speed of light, Einstein's special theory of relativity applies. B. Momentum is conserved under well-defined conditions. A change in momentum occurs when a net force is applied to an object over a time interval. C. The Law of Universa ...
... change in motion. When objects travel at speeds comparable to the speed of light, Einstein's special theory of relativity applies. B. Momentum is conserved under well-defined conditions. A change in momentum occurs when a net force is applied to an object over a time interval. C. The Law of Universa ...
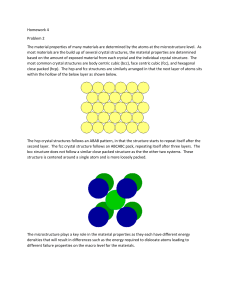
HW4P1 - Ewp.rpi.edu
... close packed (hcp). The hcp and fcc structures are similarly arranged in that the next layer of atoms sits within the hollow of the below layer as shown below. ...
... close packed (hcp). The hcp and fcc structures are similarly arranged in that the next layer of atoms sits within the hollow of the below layer as shown below. ...
Determining Krypton Concentration is Xenon
... Many contributions are continually made to the theory of dark matter, and today it is widely accepted that about 74 of the universe is dark energy, 22 is dark matter, and about 4 is visible matter which we actually see. Properties:The theory of dark matter has been one which has been readily accepte ...
... Many contributions are continually made to the theory of dark matter, and today it is widely accepted that about 74 of the universe is dark energy, 22 is dark matter, and about 4 is visible matter which we actually see. Properties:The theory of dark matter has been one which has been readily accepte ...
chemistry - billpalmer
... 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
... 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
chemical change
... o ex - a single substance breaking down into two or more other substances (hydrogen peroxide breaking into water and oxygen) o ex - two or more substances combine to form different substances (photosynthesis – several compounds combined with energy, produce new substances) o ex - vapors from glue re ...
... o ex - a single substance breaking down into two or more other substances (hydrogen peroxide breaking into water and oxygen) o ex - two or more substances combine to form different substances (photosynthesis – several compounds combined with energy, produce new substances) o ex - vapors from glue re ...
Plasma and Flames - Coalition for Plasma Science
... balance the positive charge. But if the gas temperature is high enough, particle collisions can remove some electrons from atoms, resulting in a mixture of freely moving electrons and the atoms from which they were stripped. Those atoms, which are left A natural gas stovetop flame. with an excess po ...
... balance the positive charge. But if the gas temperature is high enough, particle collisions can remove some electrons from atoms, resulting in a mixture of freely moving electrons and the atoms from which they were stripped. Those atoms, which are left A natural gas stovetop flame. with an excess po ...
Teachers` Notes
... Plasma is a mix of charged particles. It’s found a lot in our universe – in lightning, stars and even in plasma TVs in our homes. The plasma has the appearance of beams of coloured light reaching from the centre electrode to the edges of the glass ball. It looks a lot light lightning because it’s es ...
... Plasma is a mix of charged particles. It’s found a lot in our universe – in lightning, stars and even in plasma TVs in our homes. The plasma has the appearance of beams of coloured light reaching from the centre electrode to the edges of the glass ball. It looks a lot light lightning because it’s es ...
Practice Exam 3 - University of Missouri
... C2H2(g) is a. 2 C(s) + H2(g) → C2H2(g) b. 2 C(g) + 2H(g) → C2H2(g) c. 2 C2(g) + 2H(g) → C2H2(g) d. C2H6(g) → C2H2(g) + H2 e. none of the above 8. Which of the following has a standard molar enthalpy of formation of zero at 25° and 1 atm pressure? a. CO2(g) b. H2O(l) c. Zn(s) d. NO(g) ...
... C2H2(g) is a. 2 C(s) + H2(g) → C2H2(g) b. 2 C(g) + 2H(g) → C2H2(g) c. 2 C2(g) + 2H(g) → C2H2(g) d. C2H6(g) → C2H2(g) + H2 e. none of the above 8. Which of the following has a standard molar enthalpy of formation of zero at 25° and 1 atm pressure? a. CO2(g) b. H2O(l) c. Zn(s) d. NO(g) ...
Standard 4 notes
... Chemical energy can also be changed to electrical energy. When an ionic compound forms, one element donates its electrons to another element. If we separate the element that is donating from the element that is accepting and connect them with a wire, then the electrons have to go through the wire to ...
... Chemical energy can also be changed to electrical energy. When an ionic compound forms, one element donates its electrons to another element. If we separate the element that is donating from the element that is accepting and connect them with a wire, then the electrons have to go through the wire to ...
Chemistry Mid-Term Review Guide
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
... • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).