Introduction to Atoms and the Periodic Table
... Define electron, proton, neutron in terms of mass, charge, location in the atom, and the affect they have to the identity of an atom Determine number of protons, electrons, neutrons, valence electrons, mass number, and atomic number for any element using the periodic table Determine the elements and ...
... Define electron, proton, neutron in terms of mass, charge, location in the atom, and the affect they have to the identity of an atom Determine number of protons, electrons, neutrons, valence electrons, mass number, and atomic number for any element using the periodic table Determine the elements and ...
Note
... excited by radiation such as charged particles and subsequently emit pulses of light. • The substances used in the scintillation detector are – inorganic (e.g., sodium iodide or lithium iodide) or ...
... excited by radiation such as charged particles and subsequently emit pulses of light. • The substances used in the scintillation detector are – inorganic (e.g., sodium iodide or lithium iodide) or ...
Session 36 - Iowa State University
... (d) If the final volume is 1L, calculate the number of gas molecules involved in the compression. ...
... (d) If the final volume is 1L, calculate the number of gas molecules involved in the compression. ...
durfee high school science department
... 0002 Hand lab on physical and chemical properties and changes discuss pre-lab questions ticket to entry. Introduce Atomic Structure, teaching students about atomic number and atomic mass ...
... 0002 Hand lab on physical and chemical properties and changes discuss pre-lab questions ticket to entry. Introduce Atomic Structure, teaching students about atomic number and atomic mass ...
File - Mr. Stewart`s Physical Science
... Identify heterogeneous and homogeneous mixtures. Design separation processes bases on properties (e.g. magnetism, solubility, density, boiling point, and properties that lend themselves to mechanical sorting). P.12.A.4 Students know atoms bond with one another by transferring or sharing electron ...
... Identify heterogeneous and homogeneous mixtures. Design separation processes bases on properties (e.g. magnetism, solubility, density, boiling point, and properties that lend themselves to mechanical sorting). P.12.A.4 Students know atoms bond with one another by transferring or sharing electron ...
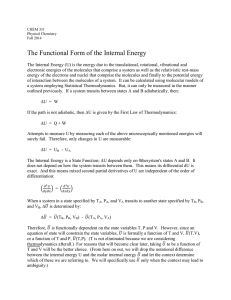
The state of a simple compressible system is completely specified by
... The state of a system is like its CONDITION, and is defined by a set of properties. Why do we need to know the state of a system? 1. Primarily, calculating energy transfer requires knowledge of the change in the state of a system (i.e., beginning and ending states) Any system has numerous properties ...
... The state of a system is like its CONDITION, and is defined by a set of properties. Why do we need to know the state of a system? 1. Primarily, calculating energy transfer requires knowledge of the change in the state of a system (i.e., beginning and ending states) Any system has numerous properties ...
Name - cloudfront.net
... If heat is released by a chemical system, an equal amount of heat will be ____. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of that material? 64. When energy is changed from one form to another, ____. 65. What happens to the energy produ ...
... If heat is released by a chemical system, an equal amount of heat will be ____. By what quantity must the heat capacity (J/oC) of an object be divided to obtain the specific heat (J/goC) of that material? 64. When energy is changed from one form to another, ____. 65. What happens to the energy produ ...
Exam 3 review - Iowa State University
... a. In diamond, the carbon atoms are bonded together in a three-dimensional tetrahedral lattice. b. Graphite has similar properties to diamond because they are both made of only carbon atoms. c. Diamond is very hard and inert because of strong covalent bonds holding all the atoms together. d. In grap ...
... a. In diamond, the carbon atoms are bonded together in a three-dimensional tetrahedral lattice. b. Graphite has similar properties to diamond because they are both made of only carbon atoms. c. Diamond is very hard and inert because of strong covalent bonds holding all the atoms together. d. In grap ...
Unit 9 Review
... _______________ proportional provided all other factors remain constant. Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly propo ...
... _______________ proportional provided all other factors remain constant. Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly propo ...
Properties of Ionic and Covalent Substances
... If an atom gains electrons it forms a negative ion (anion), and if it loses electrons it forms a positive ion (cation). Negative and positive ions attract each other and form “ionic bonds”. Ionic bonds form between metals and nonmetals when electrons are transferred. All ionic compounds have a solid ...
... If an atom gains electrons it forms a negative ion (anion), and if it loses electrons it forms a positive ion (cation). Negative and positive ions attract each other and form “ionic bonds”. Ionic bonds form between metals and nonmetals when electrons are transferred. All ionic compounds have a solid ...
Corrosion Glossary - N Nanometer: abbreviated "nm", a unit of
... material, usually ammonia or molten cyanide of appropriate composition. Quenching is not required to produce a hard case. Nitrocarburizing: any of several processes in which both nitrogen and carbon are absorbed into the surface layers of a ferrous material at temperatures below the lower critical t ...
... material, usually ammonia or molten cyanide of appropriate composition. Quenching is not required to produce a hard case. Nitrocarburizing: any of several processes in which both nitrogen and carbon are absorbed into the surface layers of a ferrous material at temperatures below the lower critical t ...
Chapter 1 Matter and Change
... least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties ...
... least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties ...
FORM 1 GEOGRAPHY REVISION GRID
... State that during a chemical change a new substance is made Recall the differences between a chemical and a physical change ...
... State that during a chemical change a new substance is made Recall the differences between a chemical and a physical change ...
Regents Chemistry
... Determine how soluble a compound is at a given temperature using the solubility traces found in Table G o use solubility curves to predict how much water is required to dissolve a given amount of solute at a given temp or how much solute will dissolve in a given amount of water o be able to predict ...
... Determine how soluble a compound is at a given temperature using the solubility traces found in Table G o use solubility curves to predict how much water is required to dissolve a given amount of solute at a given temp or how much solute will dissolve in a given amount of water o be able to predict ...
Elements compounds and mixtures
... together to form large crystal lattices. No individual molecules can be distinguished. Examples include SiO2 (quartz). Corundum (Al2O3) also forms these, even though Al is considered a metal. Network solids are among the hardest materials known. They have extremely high melting points and do not con ...
... together to form large crystal lattices. No individual molecules can be distinguished. Examples include SiO2 (quartz). Corundum (Al2O3) also forms these, even though Al is considered a metal. Network solids are among the hardest materials known. They have extremely high melting points and do not con ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).