1 Dark Matter as a consequence of electric charge non
... charged and neutral particles. In accordance with what is known at present about inflation charged particles disappeared during inflation. This observation due initially to Guth [2] has remained unchallenged by the subsequent formulations of inflation. From the point of view of the early Universe th ...
... charged and neutral particles. In accordance with what is known at present about inflation charged particles disappeared during inflation. This observation due initially to Guth [2] has remained unchallenged by the subsequent formulations of inflation. From the point of view of the early Universe th ...
a. Matter First Day of Class
... Chemical Change alters the chemical composition of the substance 2H2O ...
... Chemical Change alters the chemical composition of the substance 2H2O ...
Unit 2 Change Occurs: Investigating Matter Lesson 2
... thermometers. 2.4B Measure and compare objects using non-standard units that approximate metric units. 2.5A Classify matter by physical properties, including shape, relative mass, relative temperature, texture, flexibility, and whether material is a solid or liquid 2.5B Compare changes in materials ...
... thermometers. 2.4B Measure and compare objects using non-standard units that approximate metric units. 2.5A Classify matter by physical properties, including shape, relative mass, relative temperature, texture, flexibility, and whether material is a solid or liquid 2.5B Compare changes in materials ...
Matter - cloudfront.net
... 1. Words that describe matter (adjectives) 2. Physical Properties- a property that can be observed and measured without 3. changing the material’s composition. - Examples- color, hardness, m.p., b.p. 4. Chemical Properties- a property that can only be observed by changing the composition of the mate ...
... 1. Words that describe matter (adjectives) 2. Physical Properties- a property that can be observed and measured without 3. changing the material’s composition. - Examples- color, hardness, m.p., b.p. 4. Chemical Properties- a property that can only be observed by changing the composition of the mate ...
1 CHME 401 CHEMICAL ENGINEERING
... 3) Turn compressor (P1) on set air flow rate to 30 L/min 4) Feed CO2 set regulator to 0.5 and flow rate to 15 L/min 5) Turn pump (G1) on set flow rate of the water to 200 L/h 6) Wait 15 min until steady state conditions are reached 7) Take samples from V4 (column bottom) and V6 (feed tank) 8) Analyz ...
... 3) Turn compressor (P1) on set air flow rate to 30 L/min 4) Feed CO2 set regulator to 0.5 and flow rate to 15 L/min 5) Turn pump (G1) on set flow rate of the water to 200 L/h 6) Wait 15 min until steady state conditions are reached 7) Take samples from V4 (column bottom) and V6 (feed tank) 8) Analyz ...
Chapter 13…States of Matter
... 3. Solvent: does the dissolving 4. Miscible: liquids that are capable of dissolving each other 5. Dilute: when a solution has a low concentration of solute 6. Concentrated: when a solution has a high concentration of solute 7. Saturated solution: cannot hold any more of a given solute at a given tem ...
... 3. Solvent: does the dissolving 4. Miscible: liquids that are capable of dissolving each other 5. Dilute: when a solution has a low concentration of solute 6. Concentrated: when a solution has a high concentration of solute 7. Saturated solution: cannot hold any more of a given solute at a given tem ...
Chapter 2 - Saint Joseph High School
... – All materials are made of matter (solid, liquid, gas) ...
... – All materials are made of matter (solid, liquid, gas) ...
Periodic Table Test – Study Guide What state of matter are almost all
... What state of matter are almost all metals in at room temperature? Solid, liquid, or gas? What state of matter are nonmetals in at room temperature? Solid, liquid, or gas? What is similar for elements in a group/family on the periodic table? (2 things) number of valence e-, properties/reactivity Wha ...
... What state of matter are almost all metals in at room temperature? Solid, liquid, or gas? What state of matter are nonmetals in at room temperature? Solid, liquid, or gas? What is similar for elements in a group/family on the periodic table? (2 things) number of valence e-, properties/reactivity Wha ...
Physical Science (Properties of Matter)
... Mixtures are materials composed of two or more substances that retain their separate atomic compositions, even when mixed (e.g., water and sugar can be mixed together thoroughly at the molecular level but the water particles and sugar particles remain separate). Elements are organized into groups ba ...
... Mixtures are materials composed of two or more substances that retain their separate atomic compositions, even when mixed (e.g., water and sugar can be mixed together thoroughly at the molecular level but the water particles and sugar particles remain separate). Elements are organized into groups ba ...
Semester II Review
... • Hydrogen gas reacts with nitrogen gas to form ammonia gas. – Write and balance the equation – 3H2 + N2 2NH3 – Determine the Limiting reactant if 3.50 g of hydrogen reacts with 42.0 g of nitrogen. – What is the theoretical yield of the above problem? 39.4 g NH3 – What is the % yield if the reacti ...
... • Hydrogen gas reacts with nitrogen gas to form ammonia gas. – Write and balance the equation – 3H2 + N2 2NH3 – Determine the Limiting reactant if 3.50 g of hydrogen reacts with 42.0 g of nitrogen. – What is the theoretical yield of the above problem? 39.4 g NH3 – What is the % yield if the reacti ...
Chapter 2 Properties of Matter
... of a substance does not change; the density of lead is 11.3 g/cm³; even if we double or half the amount, a substance’s density does not change ...
... of a substance does not change; the density of lead is 11.3 g/cm³; even if we double or half the amount, a substance’s density does not change ...
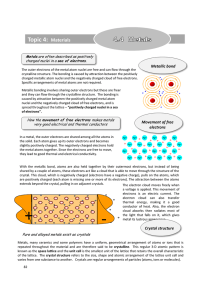
Topic 4: Materials - Education Umbrella
... shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), pulls on the atoms, which are positively charged (each atom is missing one or more of its electrons). T ...
... shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), pulls on the atoms, which are positively charged (each atom is missing one or more of its electrons). T ...
Describing Matter from Text
... In the late 1600s, experiments by the earliest chemists began to show that matter was made up of many more than four elements. Now, scientists know that all matter in the universe is made of slightly more than 100 different substances, still called elements. An element is a pure substance that canno ...
... In the late 1600s, experiments by the earliest chemists began to show that matter was made up of many more than four elements. Now, scientists know that all matter in the universe is made of slightly more than 100 different substances, still called elements. An element is a pure substance that canno ...
a4. Which of the following is the best description of a mineral? A
... a4. Which of the following is the best description of a mineral? A. pure, solid, crystalline, nonliving, natural B. pure, solid or liquid, inorganic, natural C. pure, solid, man-made or natural, non-living D. mixture, solid, non-crystalline, natural ...
... a4. Which of the following is the best description of a mineral? A. pure, solid, crystalline, nonliving, natural B. pure, solid or liquid, inorganic, natural C. pure, solid, man-made or natural, non-living D. mixture, solid, non-crystalline, natural ...
phase diagrams and IMF
... 4.) Use the phase diagram for water (above). It is not drawn to scale. The triple point occurs at 0.0060 atm and 0.01oC. Using this knowledge, answer the following questions: a. Label the triple point – use this as a reference point for the following questions (put a dot ...
... 4.) Use the phase diagram for water (above). It is not drawn to scale. The triple point occurs at 0.0060 atm and 0.01oC. Using this knowledge, answer the following questions: a. Label the triple point – use this as a reference point for the following questions (put a dot ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).