This is Most of an Old Exam
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
... Cellular oxidation of food fuels is the immediate source of electrons for oxidative phosphorylation. B. In oxidative phosphorylation, both the electron transport proteins and the ATP synthase molecules are in the same membrane. C. NAD+ and FAD+ are hydrogen carrier molecules. NAD+ can carry one hydr ...
Enzymes & Energy
... G6P is isomerized to form fructose 6-phosphate (F6P). It is phosphorylated again to form fructose 1,6diphosphate by the action of phosphofructokinase. Primes the process by providing activation energy At this point, two molecules of ATP have been consumed ...
... G6P is isomerized to form fructose 6-phosphate (F6P). It is phosphorylated again to form fructose 1,6diphosphate by the action of phosphofructokinase. Primes the process by providing activation energy At this point, two molecules of ATP have been consumed ...
Krebs Cycle - WordPress.com
... your cells need to make the most of their ATP The molecules of electron transport chains are built into the inner membranes of mitochondria The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the ...
... your cells need to make the most of their ATP The molecules of electron transport chains are built into the inner membranes of mitochondria The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the ...
Reading Guide
... 15. Enzymes called ______________________ oppose the action of kinases, turning off glycogen degradation and turning on glycogen synthesis. 16. Liver cells respond to glucagon by _________________________. 17. Muscle does not respond to glucagon, but does respond to ______________________ by releasi ...
... 15. Enzymes called ______________________ oppose the action of kinases, turning off glycogen degradation and turning on glycogen synthesis. 16. Liver cells respond to glucagon by _________________________. 17. Muscle does not respond to glucagon, but does respond to ______________________ by releasi ...
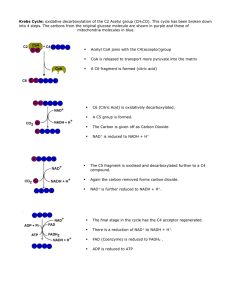
Krebs Cycle
... The krebs cycle is an example of the metabolic cycles mentioned in section 7.6.1 . Each step in the cycle requires enzymes to reduce the activation energy. The reactions all take place in the matrix of the mitochondria and are usually represented as a circular diagram. Try to overcome the idea that ...
... The krebs cycle is an example of the metabolic cycles mentioned in section 7.6.1 . Each step in the cycle requires enzymes to reduce the activation energy. The reactions all take place in the matrix of the mitochondria and are usually represented as a circular diagram. Try to overcome the idea that ...
A2 Respiration test
... enters the Kreb’s cycle which occurs in the …………………………………………. of the matrix ...
... enters the Kreb’s cycle which occurs in the …………………………………………. of the matrix ...
Document
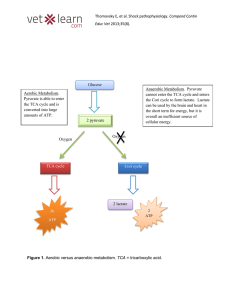
... d. Anaerobes produces an extra FADH2 during the TCA cycle ______________________________________________________________________________________________ _____________________________________________________________________________________________ ...
... d. Anaerobes produces an extra FADH2 during the TCA cycle ______________________________________________________________________________________________ _____________________________________________________________________________________________ ...
File
... Catabolic reactions are the breakdown of large molecules to smaller or simpler molecules Energy is released in this process → Exergonic ...
... Catabolic reactions are the breakdown of large molecules to smaller or simpler molecules Energy is released in this process → Exergonic ...
A mutant defective in enzyme
... 4. All the following are common properties of enzymes except: (a) their active sites contain large hydrophobic pockets. (b) they usually bind their substrates through multiple weak noncovalent interactions. (c) they exhibit saturation kinetics at high substrate concentrations. (e)they have a high de ...
... 4. All the following are common properties of enzymes except: (a) their active sites contain large hydrophobic pockets. (b) they usually bind their substrates through multiple weak noncovalent interactions. (c) they exhibit saturation kinetics at high substrate concentrations. (e)they have a high de ...
Cellular Respiration Chapter 9
... The cell can use Fermentation instead!! Occurs in the Cytoplasm Just like glycolysis!! Fermentation A series of reactions that convert NADH (from glycolysis) back into NAD allowing glycolysis to keep producing a small amount of ATP ...
... The cell can use Fermentation instead!! Occurs in the Cytoplasm Just like glycolysis!! Fermentation A series of reactions that convert NADH (from glycolysis) back into NAD allowing glycolysis to keep producing a small amount of ATP ...
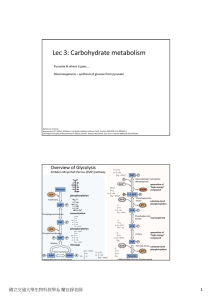
Lec 3: Carbohydrate metabolism
... With exception of PGK step (Step 7), all the other steps associated with ATP consumption or generation are regulated steps in the pathway. Why? These reactions have large decrease in ΔG, which makes them irreversible steps in vivo. Recall that irreversible steps are the place for metabolic co ...
... With exception of PGK step (Step 7), all the other steps associated with ATP consumption or generation are regulated steps in the pathway. Why? These reactions have large decrease in ΔG, which makes them irreversible steps in vivo. Recall that irreversible steps are the place for metabolic co ...
Multiple Choice Review- Photosynthesis and Cellular Respiration
... a. The addition of electrons to a molecule b. The addition of protons to a molecule c. The loss of electrons from a molecule d. The loss of protons from a molecule 2. What molecules are necessary for aerobic cellular respiration? a. Glucose and Oxygen b. Glucose and Carbon Dioxide c. Carbon Dioxide ...
... a. The addition of electrons to a molecule b. The addition of protons to a molecule c. The loss of electrons from a molecule d. The loss of protons from a molecule 2. What molecules are necessary for aerobic cellular respiration? a. Glucose and Oxygen b. Glucose and Carbon Dioxide c. Carbon Dioxide ...
Fructose-1,6 - LSU School of Medicine
... Phosphoenolpyruvate carboxykinase is regulated at the level of ...
... Phosphoenolpyruvate carboxykinase is regulated at the level of ...
Photosynthesis and Respiration 1. What are the three parts of an
... CO2 + H2O + sun energy C6H12O6 + O2 a. Does it occur in plants, animals or both? ...
... CO2 + H2O + sun energy C6H12O6 + O2 a. Does it occur in plants, animals or both? ...
Exam 3 - Chemistry Courses: About
... A. ____________ A compound with a relatively higher reduction potential is more likely to accept electrons in a redox reaction. B. ____________ The in vivo P:O ratio for NADH oxidation in mammals is about 1.5. C. ____________ Chemically, the reaction catalyzed by -ketogluterate dehydrogenase is mor ...
... A. ____________ A compound with a relatively higher reduction potential is more likely to accept electrons in a redox reaction. B. ____________ The in vivo P:O ratio for NADH oxidation in mammals is about 1.5. C. ____________ Chemically, the reaction catalyzed by -ketogluterate dehydrogenase is mor ...
Cellular Respiration (Chapter 8) Outline The Killers Are Coming
... 2. During each turn of the cycle, three carbon atoms enter (as pyruvate) and three leave as three carbon dioxide molecules. ...
... 2. During each turn of the cycle, three carbon atoms enter (as pyruvate) and three leave as three carbon dioxide molecules. ...
Document
... NADP+ picks up two high-energy electrons, along with a hydrogen ion (H+). It is then converted into NADPH. The NADPH can then carry the high-energy electrons to be used in chemical reactions elsewhere in the cell. ...
... NADP+ picks up two high-energy electrons, along with a hydrogen ion (H+). It is then converted into NADPH. The NADPH can then carry the high-energy electrons to be used in chemical reactions elsewhere in the cell. ...
Study Guide for Cellular Respiration Answers
... is then reduced by NADH to ethyl alcohol. This then regenerates NAD+ by transferring electrons from NADH to pyruvate. 13. Lactic acid fermentation is anaerobic respiration in which Glycolysis occurs but due to the lack of oxygen this regenerates NAD+ which is needed for Glycolysis. No CO2 is produce ...
... is then reduced by NADH to ethyl alcohol. This then regenerates NAD+ by transferring electrons from NADH to pyruvate. 13. Lactic acid fermentation is anaerobic respiration in which Glycolysis occurs but due to the lack of oxygen this regenerates NAD+ which is needed for Glycolysis. No CO2 is produce ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑