Macromolecules
... monomer for carbohydrates is glucose. It is a simple sugar. This is the form the cells in our body can break down. Other common monomers are fructose and galactose. ...
... monomer for carbohydrates is glucose. It is a simple sugar. This is the form the cells in our body can break down. Other common monomers are fructose and galactose. ...
Acetyl CoA - WordPress.com
... this reaction is catalyzed by pyruvate dehydrogenase the NAD+ required for this reaction and for the oxidation of glyceraldehyde 3 phosphate is regenerated when NADH ultimately transfers its electrons to O2 through the electron transport chain in mitochondria In the overall reaction, the carboxyl gr ...
... this reaction is catalyzed by pyruvate dehydrogenase the NAD+ required for this reaction and for the oxidation of glyceraldehyde 3 phosphate is regenerated when NADH ultimately transfers its electrons to O2 through the electron transport chain in mitochondria In the overall reaction, the carboxyl gr ...
Mag-Malate Magnesium Amino Acid Chelate
... Magnesium: Magnesium is a trace mineral that is essential for energy metabolism. It is required as a cofactor of enzymes in all three modes of ATP production in the muscle. In immediate, high intensity energy production, magnesium serves as an essential enzyme cofactor in both the conversion of ADP ...
... Magnesium: Magnesium is a trace mineral that is essential for energy metabolism. It is required as a cofactor of enzymes in all three modes of ATP production in the muscle. In immediate, high intensity energy production, magnesium serves as an essential enzyme cofactor in both the conversion of ADP ...
October 24 AP Biology - John D. O`Bryant School of Math & Science
... Glycolysis 2 ATP Kreb’s cycle 2 ATP Life takes a lot of energy to run, need to extract more energy than 4 ATP! There’s got to be a better way! ...
... Glycolysis 2 ATP Kreb’s cycle 2 ATP Life takes a lot of energy to run, need to extract more energy than 4 ATP! There’s got to be a better way! ...
Carbohydrate Metabolism
... Digestion of carbohydrate by salivary α -amylase (ptylin) in the mouth: A. This enzyme is produced by salivary glands. Its optimum pH is 6.7. B. It is activated by chloride ions (cl-). C. It acts on cooked starch and glycogen breaking α 1-4 bonds, converting them into maltose [a disaccharide contain ...
... Digestion of carbohydrate by salivary α -amylase (ptylin) in the mouth: A. This enzyme is produced by salivary glands. Its optimum pH is 6.7. B. It is activated by chloride ions (cl-). C. It acts on cooked starch and glycogen breaking α 1-4 bonds, converting them into maltose [a disaccharide contain ...
Citric Acid Cycle - chem.uwec.edu - University of Wisconsin
... label was in the released CO2. Why were the early investigators of the citric acid cycle surprised that all the label emerged in the CO2? ...
... label was in the released CO2. Why were the early investigators of the citric acid cycle surprised that all the label emerged in the CO2? ...
Electron Transport and oxidative phosphorylation (ATP Synthesis)
... Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Abir Alghanouchi Biochemistry department Sciences college ...
... Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Abir Alghanouchi Biochemistry department Sciences college ...
Lecture 4 - Citric Acid Cycle 1 2 3 4 - chem.uwec.edu
... Citric acid cycle is also an important source of precursors Two of the intermediates are only one step away from an amino acid One of the intermediates is used in the synthesis of ...
... Citric acid cycle is also an important source of precursors Two of the intermediates are only one step away from an amino acid One of the intermediates is used in the synthesis of ...
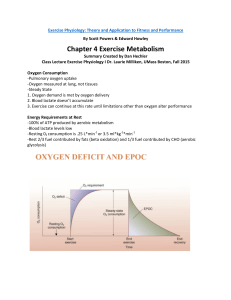
Chapter 4 Exercise Metabolism
... -Blood lactate levels low -Resting 02 consumption is .25 L*min-1 or 3.5 ml*kg-1*min-1 -Rest 2/3 fuel contributed by fats (beta oxidation) and 1/3 fuel contributed by CHO (aerobic glycolysis) ...
... -Blood lactate levels low -Resting 02 consumption is .25 L*min-1 or 3.5 ml*kg-1*min-1 -Rest 2/3 fuel contributed by fats (beta oxidation) and 1/3 fuel contributed by CHO (aerobic glycolysis) ...
Chapter 17 – Amino Acid Metabolism
... Common in newborns as fetal hemoglobin is broken down and replaced by adult hemoglobin. ...
... Common in newborns as fetal hemoglobin is broken down and replaced by adult hemoglobin. ...
File
... reactions • Releases it within seconds for physiological work • Holds energy in covalent bonds – Second and third phosphate groups have high energy bonds (~) – Most energy transfers to and from ATP involve adding or removing the third phosphate ...
... reactions • Releases it within seconds for physiological work • Holds energy in covalent bonds – Second and third phosphate groups have high energy bonds (~) – Most energy transfers to and from ATP involve adding or removing the third phosphate ...
1 Glucose: evolution`s favorite flavor… In any metabolism course
... carbonyl to make an adduct that simply rearranges the extant atoms, as shown in the picture of the generic reaction. That is a hemiacetal, and this adduct is in equilibrium with the free alcohol and aldehyde; hence the double arrow. It is through this reaction that linear glucose forms the familiar ...
... carbonyl to make an adduct that simply rearranges the extant atoms, as shown in the picture of the generic reaction. That is a hemiacetal, and this adduct is in equilibrium with the free alcohol and aldehyde; hence the double arrow. It is through this reaction that linear glucose forms the familiar ...
Cellular Respiration
... course. Instead, if you swallow some glucose, enzymes in your cells will lower the barrier of activation energy allowing the sugar to be oxidized in a series of steps. ...
... course. Instead, if you swallow some glucose, enzymes in your cells will lower the barrier of activation energy allowing the sugar to be oxidized in a series of steps. ...
Cellular Respiration Notes (8.3)
... Which represents the general sequence of cellular respiration? A. TCA cycle chemiosmosis glycolysis B. glycolysis Krebs ...
... Which represents the general sequence of cellular respiration? A. TCA cycle chemiosmosis glycolysis B. glycolysis Krebs ...
T cell Metabolism–Regulating Energy
... and CD28 lead to direct phosphorylation of phosphatidylinositol-4,5bisphosphate (PIP2) by phosphoinositide 3-kinase (PI3K) which leads to increased levels of phosphatidylinositol-3,4,5-trisphosphate (PIP3). AKT translocates to the plasma membrane by binding PIP3 via its PHdomain, where it can be pho ...
... and CD28 lead to direct phosphorylation of phosphatidylinositol-4,5bisphosphate (PIP2) by phosphoinositide 3-kinase (PI3K) which leads to increased levels of phosphatidylinositol-3,4,5-trisphosphate (PIP3). AKT translocates to the plasma membrane by binding PIP3 via its PHdomain, where it can be pho ...
Energy For Muscular Activity
... b) sufficient oxygen is supplied to the mitochondria c) enzymes or intermediate products do not limit the Kreb’s cycle ...
... b) sufficient oxygen is supplied to the mitochondria c) enzymes or intermediate products do not limit the Kreb’s cycle ...
File
... A chemical reaction may involve the joining together of simple molecules into more complex ones or the splitting of complex molecules into simpler ones. Either way, energy is required to initially break the bonds (activation energy) in the reactants to form an unstable compound. The molecules are in ...
... A chemical reaction may involve the joining together of simple molecules into more complex ones or the splitting of complex molecules into simpler ones. Either way, energy is required to initially break the bonds (activation energy) in the reactants to form an unstable compound. The molecules are in ...
2. Organic Compounds and the Four Biomolec
... amino group (which is basic) and an acid group. Proteins consist of long chains of amino acids, with the acid group of one bonded to the amino group of the next. There are 20 different kinds of amino acids in proteins. Each one has a functional group (the “R group”) attached to it. Different R group ...
... amino group (which is basic) and an acid group. Proteins consist of long chains of amino acids, with the acid group of one bonded to the amino group of the next. There are 20 different kinds of amino acids in proteins. Each one has a functional group (the “R group”) attached to it. Different R group ...
Cellular Respiration and Photosynthesis
... Inner membrane proteins reduced by NADH and FADH2 AcetylCoA broken down further, releasing two CO2 molecules; C electrons and H sequestered by NADH and FADH2 Oxygen reduced by electrons from inner membrane proteins; D binds with 2 protons and released as waste H2O E Glucose hydrolyzed into two pyruv ...
... Inner membrane proteins reduced by NADH and FADH2 AcetylCoA broken down further, releasing two CO2 molecules; C electrons and H sequestered by NADH and FADH2 Oxygen reduced by electrons from inner membrane proteins; D binds with 2 protons and released as waste H2O E Glucose hydrolyzed into two pyruv ...
Light-independent reactions
... The enzyme RuBisCO (short for ribulose biphosphate carboxylase-oxygenase) is the most abundant enzyme on earth, as it makes approximately 50% of leaf protein. It is of upmost importance to life. Although you can see that the Calvin cycle uses RuBisCO to combine a molecule of RuBP and carbon dioxide, ...
... The enzyme RuBisCO (short for ribulose biphosphate carboxylase-oxygenase) is the most abundant enzyme on earth, as it makes approximately 50% of leaf protein. It is of upmost importance to life. Although you can see that the Calvin cycle uses RuBisCO to combine a molecule of RuBP and carbon dioxide, ...
Marvelous Macromolecules - Pregitzersninjascienceclasses
... Six carbons – hexose Five carbons - pentose Three carbons - triose ...
... Six carbons – hexose Five carbons - pentose Three carbons - triose ...
Ch. 6 PPT
... • In chemiosmosis, the H+ diffuses back through the inner membrane through ATP synthase complexes ...
... • In chemiosmosis, the H+ diffuses back through the inner membrane through ATP synthase complexes ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑