Supplementary material for table on macromolecular cell
... blocks synthesis, transport processes etc. Those were accounted for separately and as shown for example by A. H. Stouthamer 1973 (BNID 104848, assuming it refers to cell dry weight), and amount to another ≈ 7×109 ATP per 1 μm3 cell volume. ...
... blocks synthesis, transport processes etc. Those were accounted for separately and as shown for example by A. H. Stouthamer 1973 (BNID 104848, assuming it refers to cell dry weight), and amount to another ≈ 7×109 ATP per 1 μm3 cell volume. ...
Enzymes: Biological Catalysts
... DNA Polymerase: Adds nucleic acid bases to growing DNA strands during DNA replication. Kinase: attaches phosphate groups; ATP production ...
... DNA Polymerase: Adds nucleic acid bases to growing DNA strands during DNA replication. Kinase: attaches phosphate groups; ATP production ...
Metabolism
... free energy of hydrolysis: • 1. The negative charges of the phosphates repel each other. • 2. The products ADP and P form "resonance hybrids," which means that they can share electrons in ways to reduce the energy state. • 3. ADP and ATP have the proper configurations to be accepted by enzymes that ...
... free energy of hydrolysis: • 1. The negative charges of the phosphates repel each other. • 2. The products ADP and P form "resonance hybrids," which means that they can share electrons in ways to reduce the energy state. • 3. ADP and ATP have the proper configurations to be accepted by enzymes that ...
Chapter 8 – an introduction to metabolism
... 7. Name the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where s ...
... 7. Name the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where s ...
Topic 2 - Wolfgang Hess
... multiple uptake systems for combined N-sources and of enzymes involved in specific routes of the N-assimilation. The Nand energetic status is sensed by the PII-protein which regulates the activity of the transcriptional regulator NtcA. Subsequently, NtcA controls the coordinated up-regulation of gen ...
... multiple uptake systems for combined N-sources and of enzymes involved in specific routes of the N-assimilation. The Nand energetic status is sensed by the PII-protein which regulates the activity of the transcriptional regulator NtcA. Subsequently, NtcA controls the coordinated up-regulation of gen ...
Metabolic changes in the glucose-induced apoptotic blastocyst
... these blastocysts, as demonstrated by decreased FBP levels, and the embryos’ attempts to compensate by increasing pyruvate uptake lead to severe alteration in mitochondrial physiology that result in the triggering of the apoptotic cascade. As shown in the growth factor withdrawal model (27), a deple ...
... these blastocysts, as demonstrated by decreased FBP levels, and the embryos’ attempts to compensate by increasing pyruvate uptake lead to severe alteration in mitochondrial physiology that result in the triggering of the apoptotic cascade. As shown in the growth factor withdrawal model (27), a deple ...
Lecture 26
... Citrate synthase: catalyzes the condensation of acetyl-CoA and oxaloacetate to yield citrate. Aconitase: isomerizes citrate to the easily oxidized isocitrate. Isocitrate dehydrogenase: oxidizes isocitrate to the -keto acid oxalosuccinate, coupled to NADH formation. Oxalosuccinate is then decarboxyl ...
... Citrate synthase: catalyzes the condensation of acetyl-CoA and oxaloacetate to yield citrate. Aconitase: isomerizes citrate to the easily oxidized isocitrate. Isocitrate dehydrogenase: oxidizes isocitrate to the -keto acid oxalosuccinate, coupled to NADH formation. Oxalosuccinate is then decarboxyl ...
RACC BIO Cellular respiration
... • The citric acid cycle completes the energyyielding oxidation of organic molecules • The citric acid cycle – Takes place in the matrix of the mitochondrion ...
... • The citric acid cycle completes the energyyielding oxidation of organic molecules • The citric acid cycle – Takes place in the matrix of the mitochondrion ...
02 B organic chemistry - macromolecules
... carbohydrate (in plants) - The difference between digestible (to us) starch and indigestible cellulose is… (can you see it?) [Only certain bacteria make the enzymes to digest cellulose. Generally, any animal living off grass or wood has these specific bacteria in their guts to break the cellulose in ...
... carbohydrate (in plants) - The difference between digestible (to us) starch and indigestible cellulose is… (can you see it?) [Only certain bacteria make the enzymes to digest cellulose. Generally, any animal living off grass or wood has these specific bacteria in their guts to break the cellulose in ...
Human Physiology
... one “up” and one “down” across from each other. • Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds ...
... one “up” and one “down” across from each other. • Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds ...
Jeopardy - Alfred State College intranet site
... A multi-substrate reaction in which the first product is released from the active site before the second product binds to the active site is known by this term. ...
... A multi-substrate reaction in which the first product is released from the active site before the second product binds to the active site is known by this term. ...
Energy Review Questions
... Explain how pH and temperature affect enzyme action. The activity of enzymes is strongly affected by changes in pH and temperature. Each enzyme works best at a certain pH and temperature, its activity decreasing at values above and below that point. Explain how competitive inhibition affects reactio ...
... Explain how pH and temperature affect enzyme action. The activity of enzymes is strongly affected by changes in pH and temperature. Each enzyme works best at a certain pH and temperature, its activity decreasing at values above and below that point. Explain how competitive inhibition affects reactio ...
... Choice B: ATP synthase consists of two subunits. The one in the membrane (Fo) transports the protons, the one in the mitochondrial matrix (F1) synthesizes ATP. (2 pts) The F1 domain contains three β-subunits, whose conformation depends on the relative orientation of the γ-subunit. The orientation of ...
video slide - Northwest Florida State College
... 2) All of these produce far less E for ATP production. ...
... 2) All of these produce far less E for ATP production. ...
Ch.24Pt.4_000
... •precursors in synthesis of other compounds •fuels for energy production •substrates for ketone body synthesis. Ketone bodies may be exported to other tissues: used for energy production. Some cells synthesize fatty acids for storage or ...
... •precursors in synthesis of other compounds •fuels for energy production •substrates for ketone body synthesis. Ketone bodies may be exported to other tissues: used for energy production. Some cells synthesize fatty acids for storage or ...
Biochemistry Midterm Review
... 7. Name 3 elements your body needs trace amounts of for proper functioning. The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential to the proper functioning of all living things are known as polymers or macromolecules. All of these compound ...
... 7. Name 3 elements your body needs trace amounts of for proper functioning. The four main classes of organic compounds (carbohydrates, lipids, proteins, and nucleic acids) that are essential to the proper functioning of all living things are known as polymers or macromolecules. All of these compound ...
Final Review Part I
... 83. True or false: During periods of fasting, the hormone glucagon signals to the liver to break down glycogen and release it as glucose into the blood stream. 84. True or false: The pancreas releases both insulin and glucagon. 85. Type I diabetes or type II diabetes: the failure to make insulin. 86 ...
... 83. True or false: During periods of fasting, the hormone glucagon signals to the liver to break down glycogen and release it as glucose into the blood stream. 84. True or false: The pancreas releases both insulin and glucagon. 85. Type I diabetes or type II diabetes: the failure to make insulin. 86 ...
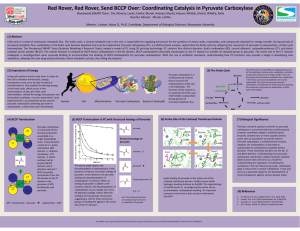
Poster
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
Fe-S
... 12e- from the oxidation of glucose are not transferred directly to O2, go to NAD+ and FAD to form 10NADH and 2FADH2 These are reoxidized, passing their electrons to the electrontransport chain to reduce O2 to H2O causing the mitochondrion to create a proton gradient. This pH gradient is used to driv ...
... 12e- from the oxidation of glucose are not transferred directly to O2, go to NAD+ and FAD to form 10NADH and 2FADH2 These are reoxidized, passing their electrons to the electrontransport chain to reduce O2 to H2O causing the mitochondrion to create a proton gradient. This pH gradient is used to driv ...
How Does Life Use Energy?
... alcohol and carbon dioxide (CO2) Conclusion: Living cells are not required for fermentation – only need some materials that were present within the cells (now known to be enzymes - proteins that act as catalysts to speed up reactions). ...
... alcohol and carbon dioxide (CO2) Conclusion: Living cells are not required for fermentation – only need some materials that were present within the cells (now known to be enzymes - proteins that act as catalysts to speed up reactions). ...
AP Biology 2015 Free-Response Questions
... 6. In an attempt to rescue a small isolated population of snakes from decline, a few male snakes from several larger populations of the same species were introduced into the population in 1992. The snakes reproduce sexually, and there are abundant resources in the environment. The figure below shows ...
... 6. In an attempt to rescue a small isolated population of snakes from decline, a few male snakes from several larger populations of the same species were introduced into the population in 1992. The snakes reproduce sexually, and there are abundant resources in the environment. The figure below shows ...
Regulation of fatty acid synthesis and degradation by the AMP
... The AMP-activated protein kinase (AMPK) is the downstream component of a kinase cascade that is activated by rising AMP and falling ATP, which together signal a fall in cellular energy status. Although it probably has many targets, two key targets are acetylCoA carboxylase-1 and -2 (ACCI and ACCZ), ...
... The AMP-activated protein kinase (AMPK) is the downstream component of a kinase cascade that is activated by rising AMP and falling ATP, which together signal a fall in cellular energy status. Although it probably has many targets, two key targets are acetylCoA carboxylase-1 and -2 (ACCI and ACCZ), ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑