Chemistry Worksheet
... 6. ____________The smallest particle of an element that retains the properties of the element. 7. ____________The particle of a compound, formed when atoms combine chemically 8. ____________Positively charged particles forming part of an atom. 9. ____________Non-charged particle in the nucleus. 10. ...
... 6. ____________The smallest particle of an element that retains the properties of the element. 7. ____________The particle of a compound, formed when atoms combine chemically 8. ____________Positively charged particles forming part of an atom. 9. ____________Non-charged particle in the nucleus. 10. ...
COVALENT BOND - hovanscience
... • Most of the compounds that make up living things contain carbon. • Carbon makes up the basic structure, or “backbone,” of these compounds. • Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. ...
... • Most of the compounds that make up living things contain carbon. • Carbon makes up the basic structure, or “backbone,” of these compounds. • Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. ...
Calculating Chemical Equations
... same nonmetal (usually in gas form) that have bonded together • Reactive nonmetals will bond with one another if no other substance is available for bonding ...
... same nonmetal (usually in gas form) that have bonded together • Reactive nonmetals will bond with one another if no other substance is available for bonding ...
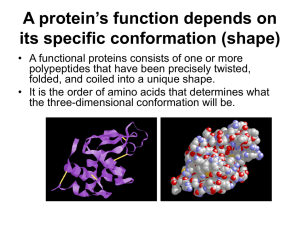
A protein’s function depends on its specific conformation
... protein’s conformation and ability to function. • In individuals with sickle cell disease, abnormal hemoglobins, oxygen-carrying proteins, develop because of a single amino acid substitution. – These abnormal hemoglobins crystallize, deforming the red blood cells and leading to clogs in tiny blood v ...
... protein’s conformation and ability to function. • In individuals with sickle cell disease, abnormal hemoglobins, oxygen-carrying proteins, develop because of a single amino acid substitution. – These abnormal hemoglobins crystallize, deforming the red blood cells and leading to clogs in tiny blood v ...
Earth`s Chemistry
... of neutrons Each additional neutron increases the mass number. Isotopes = atoms of the same element that differ from each other by mass number. ...
... of neutrons Each additional neutron increases the mass number. Isotopes = atoms of the same element that differ from each other by mass number. ...
Unit 1 PPT 3 (2biii-iv Binding and conformation)
... • Why is it important that protein production is controlled? • Why is protein structure important in relation to its function? ...
... • Why is it important that protein production is controlled? • Why is protein structure important in relation to its function? ...
Slide 1
... Proteins that are not changed or used up in the reaction – specific — will only work on limited types of substrates – limited — by their saturation – regulated — by other cellular chemicals ...
... Proteins that are not changed or used up in the reaction – specific — will only work on limited types of substrates – limited — by their saturation – regulated — by other cellular chemicals ...
File
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
Complexes_naming(download)
... (a) The structure of the porphine molecule. Loss of the two NH protons gives a planar, tetradentate 2– ligand that can bond to a metal cation. The porphyrins are derivatives of porphine in which the peripheral H atoms are replaced by various substituent groups. (b) Schematic of the planar heme group ...
... (a) The structure of the porphine molecule. Loss of the two NH protons gives a planar, tetradentate 2– ligand that can bond to a metal cation. The porphyrins are derivatives of porphine in which the peripheral H atoms are replaced by various substituent groups. (b) Schematic of the planar heme group ...
CVB101 – Lecture 3 Chemical Bonding • Chemical bonding
... The maximum amount of solute that will dissolve in a given quantity of solvent (at a specific temperature) Some compounds are very soluble e.g. NaCl o It is possible to make very concentrated solutions on NaCl Other compounds are not very soluble e.g. AgCl o If AgCl solid is placed in water, o ...
... The maximum amount of solute that will dissolve in a given quantity of solvent (at a specific temperature) Some compounds are very soluble e.g. NaCl o It is possible to make very concentrated solutions on NaCl Other compounds are not very soluble e.g. AgCl o If AgCl solid is placed in water, o ...
Exam I F'01 (1710).doc
... Darwin collected fossils of extinct species from around the world. Comparing these to living plant and animal species he discovered that: a) extinct species of a continent generally resembled the living species of that continent. b) extinct species of all continents looked generally similar to each ...
... Darwin collected fossils of extinct species from around the world. Comparing these to living plant and animal species he discovered that: a) extinct species of a continent generally resembled the living species of that continent. b) extinct species of all continents looked generally similar to each ...
A2 Module 2814: Chains, Rings and Spectroscopy
... the proteins found in living organisms. They are all α-amino acids, i.e. the NH2 and the COOH groups are attached to the same carbon atom. Their general formula is RCH(NH 2)COOH, where R represents a side-chain (not just an alkyl group). Three examples are given below: ...
... the proteins found in living organisms. They are all α-amino acids, i.e. the NH2 and the COOH groups are attached to the same carbon atom. Their general formula is RCH(NH 2)COOH, where R represents a side-chain (not just an alkyl group). Three examples are given below: ...
Ch. 7 Review Sheet w/ans
... What is the outer electron configuration of Sr? Compare Sr to Ca and Rb in atomic radius and first ionization energy. How does the Sr2+ ion compare in size to the Sr atom? To the Ba2+ ion? As successive electrons are removed from the Sr atom, where does the largest jump in ionization energy occur? H ...
... What is the outer electron configuration of Sr? Compare Sr to Ca and Rb in atomic radius and first ionization energy. How does the Sr2+ ion compare in size to the Sr atom? To the Ba2+ ion? As successive electrons are removed from the Sr atom, where does the largest jump in ionization energy occur? H ...
Diagram Sodium has 11 protons and 11 neutrons in its nucleus
... 2. (not hydrogen nor covalent) bonds are formed when one atom donates an electron to another atom, whereas (not hydrogen nor ionic) bonds are formed when two atoms share a pair of electrons. 3. An (begins with “I” and name means very similar) is a form of an element that has a different number of ne ...
... 2. (not hydrogen nor covalent) bonds are formed when one atom donates an electron to another atom, whereas (not hydrogen nor ionic) bonds are formed when two atoms share a pair of electrons. 3. An (begins with “I” and name means very similar) is a form of an element that has a different number of ne ...
Origin of Life (IB)
... such as protein and nucleic acids. a. How would this occur without enzymes? b. In experiments, polymerization does occur when solutions of monomers are dropped onto hot sand, clay or rock. ...
... such as protein and nucleic acids. a. How would this occur without enzymes? b. In experiments, polymerization does occur when solutions of monomers are dropped onto hot sand, clay or rock. ...
3 - Copley-Fairlawn City Schools
... A reagent whose anion forms a precipitate with either one or a few metal ions in the mixture will precipitate certain metals out of solution and leave the others. So a solution of Ca2+ K+, can be separated by adding a CO32- ion because this will form a precipitate with calcium ...
... A reagent whose anion forms a precipitate with either one or a few metal ions in the mixture will precipitate certain metals out of solution and leave the others. So a solution of Ca2+ K+, can be separated by adding a CO32- ion because this will form a precipitate with calcium ...
ppt - UCLA Chemistry and Biochemistry
... The symmetry (concerted) model of cooperativity Subunits can adopt one of two possible conformations: T or R. All subunits must adopt the same conformation (protein is always symmetric). Equilibrium between T and R states is influenced by ligand or modulator binding. The sequential (gradual) model ...
... The symmetry (concerted) model of cooperativity Subunits can adopt one of two possible conformations: T or R. All subunits must adopt the same conformation (protein is always symmetric). Equilibrium between T and R states is influenced by ligand or modulator binding. The sequential (gradual) model ...
Document
... Cryogenic protein storage and assessment of protein purity Flash freezing of protein for long term storage. Mass spectrometry and SDS-PAGE for determination of purity and molecular weight. Preparation of buffers for experiments in following weeks. ...
... Cryogenic protein storage and assessment of protein purity Flash freezing of protein for long term storage. Mass spectrometry and SDS-PAGE for determination of purity and molecular weight. Preparation of buffers for experiments in following weeks. ...
L. LEWIS ACID CATALYSIS
... Twenty amino acids is not enough. The breadth of chemistry handled by enzymes requires that additional chemical species be employed in catalysis. So-called cofactors are non-amino acid components of enzymes that may be either associated or bonded to proteins and contribute to rate acceleration. Roug ...
... Twenty amino acids is not enough. The breadth of chemistry handled by enzymes requires that additional chemical species be employed in catalysis. So-called cofactors are non-amino acid components of enzymes that may be either associated or bonded to proteins and contribute to rate acceleration. Roug ...
The Mac Daddies of Molecules
... They are fats,steroids,oils and waxes Examples are margarine, shortening, meats, olive oil, peanut oil Lipids are used for storing energy (why it pays to have some fat on you!) ...
... They are fats,steroids,oils and waxes Examples are margarine, shortening, meats, olive oil, peanut oil Lipids are used for storing energy (why it pays to have some fat on you!) ...
Chemical Organization of Life
... Water is a versatile solvent due to: its polarity Readily forms hydrogen bonds ...
... Water is a versatile solvent due to: its polarity Readily forms hydrogen bonds ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.