STRUCTURAL AND FUNCTIONAL MIMIC OF
... The stability constant and composition of the evolving species in aqueous solution were calculated on the basis of experimental data of pH-potentiometric titration using the Psequad and Superquad computer packages. The structures of these complexes were investigated by pH-dependent UV-Vis, CD, EPR a ...
... The stability constant and composition of the evolving species in aqueous solution were calculated on the basis of experimental data of pH-potentiometric titration using the Psequad and Superquad computer packages. The structures of these complexes were investigated by pH-dependent UV-Vis, CD, EPR a ...
honors biology - Uplift Education
... PROTEIN is a large molecule formed by linked smaller molecules called amino acids. 20 types of amino acids are found. They may be polar, non polar or electrically charged. Proteins tend to fold into compact shapes determined by how the protein’s amino acids interact with water and one another. ...
... PROTEIN is a large molecule formed by linked smaller molecules called amino acids. 20 types of amino acids are found. They may be polar, non polar or electrically charged. Proteins tend to fold into compact shapes determined by how the protein’s amino acids interact with water and one another. ...
9.1 Electron Transfer Reactions
... 5. O is usually – 2 (except for peroxides where it is – 1) 6. H is usually +1 (except for hydrides where it is – 1) 7. The periodic table can used as a guide for an atom’s oxidation number in a compound (ex: F is usually – 1, alkali metals are usually +1) ...
... 5. O is usually – 2 (except for peroxides where it is – 1) 6. H is usually +1 (except for hydrides where it is – 1) 7. The periodic table can used as a guide for an atom’s oxidation number in a compound (ex: F is usually – 1, alkali metals are usually +1) ...
College_Chemistry_Chapter_4_Study_Guide
... 6. In compounds, the elements of Group IA and Group IIA and aluminum have positive oxidation numbers numerically equal to their group number in the periodic table. ...
... 6. In compounds, the elements of Group IA and Group IIA and aluminum have positive oxidation numbers numerically equal to their group number in the periodic table. ...
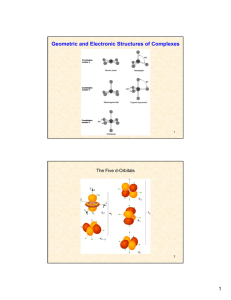
Geometric and Electronic Structures of Complexes
... (the electron “hole” can be in either of the two eg orbitals) Therefore, a tetragonal distortion of octahedral Cu(II) complexes should occur Such distortion (along the z axis) removes the degeneracy and leads to a higher CFSE The gain in CFSE is what ...
... (the electron “hole” can be in either of the two eg orbitals) Therefore, a tetragonal distortion of octahedral Cu(II) complexes should occur Such distortion (along the z axis) removes the degeneracy and leads to a higher CFSE The gain in CFSE is what ...
4 Chemistry
... Numbers that sit after and below the symbol. Represent number of atoms of that particular element. Numbers used to show how many molecules and/or compounds there are in a substance Are used to show reactions All substances needed for the reaction to take place All substances are on the left hand ...
... Numbers that sit after and below the symbol. Represent number of atoms of that particular element. Numbers used to show how many molecules and/or compounds there are in a substance Are used to show reactions All substances needed for the reaction to take place All substances are on the left hand ...
Chemistry of Life
... Enzymes • Globular proteins acting as catalysts to speed a reaction – Lowers energy of activation (EA) ...
... Enzymes • Globular proteins acting as catalysts to speed a reaction – Lowers energy of activation (EA) ...
Myoglobin and Hemoglobin - UF Macromolecular Structure Group
... Hemoglobin is an [α(2):β(2)] tetrameric hemeprotein found in erythrocytes where it is responsible for binding oxygen in the lung and transporting the bound oxygen throughout the body where it is used in aerobic metabolic pathways Each subunit of a hemoglobin tetramer has a heme prosthetic group ide ...
... Hemoglobin is an [α(2):β(2)] tetrameric hemeprotein found in erythrocytes where it is responsible for binding oxygen in the lung and transporting the bound oxygen throughout the body where it is used in aerobic metabolic pathways Each subunit of a hemoglobin tetramer has a heme prosthetic group ide ...
Chemical Reactions in Living Things
... Examples of anabolic reactions include building up starch molecules from individual glucose molecules or building up proteins from amino acids. A very important CATABOLIC reaction is respiration when glucose is oxidised to release CO2, H2O and E (ATP). ENZYMES control all these chemical reactions. E ...
... Examples of anabolic reactions include building up starch molecules from individual glucose molecules or building up proteins from amino acids. A very important CATABOLIC reaction is respiration when glucose is oxidised to release CO2, H2O and E (ATP). ENZYMES control all these chemical reactions. E ...
16 1 Stanford Notes - Mayfield City Schools
... 9. Which group of elements don’t usually form chemical bonds with other atoms? _______________ 10. What do we call the particle that is a group of atoms held together by covalent bonds? __________ 11. For each molecule listed below, tell how many of atoms of each element are in that molecule. a) ...
... 9. Which group of elements don’t usually form chemical bonds with other atoms? _______________ 10. What do we call the particle that is a group of atoms held together by covalent bonds? __________ 11. For each molecule listed below, tell how many of atoms of each element are in that molecule. a) ...
NAME REVIEW 1: JUST THE BASICS ___1) In which material are
... 20) 1) HI it is produced endothermically and that means more energy is absorbed by the breaking of bonds than is released as the new H-I polar covalent bond(s) is (are) produced. Thus HI is less stable than the reactants. 21) 3 an increase in temp favors the endo. rxn which in this case is the forwa ...
... 20) 1) HI it is produced endothermically and that means more energy is absorbed by the breaking of bonds than is released as the new H-I polar covalent bond(s) is (are) produced. Thus HI is less stable than the reactants. 21) 3 an increase in temp favors the endo. rxn which in this case is the forwa ...
chelate. In chlorophyll
... vertebrates, heme is bound to proteins forming hemoglobin. Hemoglobin CH2 H2 C H combines with oxygen in the lungs, gills, or other respiratory surfaces and CH2 COOH HOOC CH2 releases it in the tissues. In muscle cells, myoglobin, the name given to hemoglobin in muscles, stores oxygen as an electron ...
... vertebrates, heme is bound to proteins forming hemoglobin. Hemoglobin CH2 H2 C H combines with oxygen in the lungs, gills, or other respiratory surfaces and CH2 COOH HOOC CH2 releases it in the tissues. In muscle cells, myoglobin, the name given to hemoglobin in muscles, stores oxygen as an electron ...
Chapter 2: The Chemistry of Life
... 3. Nucleic Acids – made of C, H, O, P, N a. Nucleotides – 5-carbon sugar, phosphate group, and nitrogenous base b. Nucleic Acid – nucleotides joined by covalent bonds 1) Store and transmit genetic info a) RNA – Ribonucleic Acid b) DNA – Deoxyribonucleic Acid 4. Proteins – made of C, H, O, N a. Amino ...
... 3. Nucleic Acids – made of C, H, O, P, N a. Nucleotides – 5-carbon sugar, phosphate group, and nitrogenous base b. Nucleic Acid – nucleotides joined by covalent bonds 1) Store and transmit genetic info a) RNA – Ribonucleic Acid b) DNA – Deoxyribonucleic Acid 4. Proteins – made of C, H, O, N a. Amino ...
REACTIONS OF IRON(II)
... When sufficient protons have been removed the complex becomes neutral and precipitation of a hydroxide or carbonate occurs. ...
... When sufficient protons have been removed the complex becomes neutral and precipitation of a hydroxide or carbonate occurs. ...
Chapter 6, Section 3
... ◦ Substrate: substance that the enzyme breaks down Each substrate fits into the active site. (Like a lock & key) ...
... ◦ Substrate: substance that the enzyme breaks down Each substrate fits into the active site. (Like a lock & key) ...
Redox #2 Oxidation Numbers
... Redox reactions are all about electrons being transferred from one substance to another, so it would be useful if we had a system for keeping track of what gains and what loses electrons, and how many electrons are involved. We do - our record-keeping system is called Oxidation Numbers ...
... Redox reactions are all about electrons being transferred from one substance to another, so it would be useful if we had a system for keeping track of what gains and what loses electrons, and how many electrons are involved. We do - our record-keeping system is called Oxidation Numbers ...
INTODUCTION TO THE TRANSITION ELEMENTS
... Ions with partially filled d-orbitals tend to be coloured. Colour of complexes are caused by the splitting of the energy of ...
... Ions with partially filled d-orbitals tend to be coloured. Colour of complexes are caused by the splitting of the energy of ...
CHAPTER 3-Protein-In Class Activity
... Draw the structure of amino acid (aa) and label each part’s name. Draw and show how two aa bind together and go through dehydration reaction. Condensation reactions bond the carboxyl group of one amino acid to the amino group of another to form a peptide bond There are _______different amino acids e ...
... Draw the structure of amino acid (aa) and label each part’s name. Draw and show how two aa bind together and go through dehydration reaction. Condensation reactions bond the carboxyl group of one amino acid to the amino group of another to form a peptide bond There are _______different amino acids e ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.