
Transition metal Catalyzed Reactions
... order [Ar]4s23d10, this turns out to be true only for isolated metal atoms. When we put a metal ion into an electronic field (surround it with ligands), the d-orbitals drop in energy and fill first. Therefore, the organometallic chemist considers the transition metal valence electrons to all be d-el ...
... order [Ar]4s23d10, this turns out to be true only for isolated metal atoms. When we put a metal ion into an electronic field (surround it with ligands), the d-orbitals drop in energy and fill first. Therefore, the organometallic chemist considers the transition metal valence electrons to all be d-el ...
Chapter 9 ( Cyclopentadienyl)
... The changes in the neutral Fe, Co, Ni metallocenes are a direct result of going from 18e- (Fe) to 19e- (Co) to 20e- (Ni) counts. The extra electrons for the Co and Ni complexes are going into M-Cp antibonding orbitals, which are delocalized and progressively weaken the M-Cp bonding, leading to the i ...
... The changes in the neutral Fe, Co, Ni metallocenes are a direct result of going from 18e- (Fe) to 19e- (Co) to 20e- (Ni) counts. The extra electrons for the Co and Ni complexes are going into M-Cp antibonding orbitals, which are delocalized and progressively weaken the M-Cp bonding, leading to the i ...
Dr. György Keglevich e-mail: Title of the
... The purpose is to synthesize phosphoric-, phosphonic and phosphinic ester, as well as phosphine oxide intermediates by MW-assisted direct esterification, alkylating esterification, T3P-mediated esterification, or by the P-ligand-free Hirao P–C coupling methodology. The different methods should be co ...
... The purpose is to synthesize phosphoric-, phosphonic and phosphinic ester, as well as phosphine oxide intermediates by MW-assisted direct esterification, alkylating esterification, T3P-mediated esterification, or by the P-ligand-free Hirao P–C coupling methodology. The different methods should be co ...
Lecture3
... The degree of clusterification of lithium alkyls varies with the nature of the solvent between dimer (LiCH3: TMEDA) and hexamer (Li-n-C4H9: cyclohexane) ...
... The degree of clusterification of lithium alkyls varies with the nature of the solvent between dimer (LiCH3: TMEDA) and hexamer (Li-n-C4H9: cyclohexane) ...
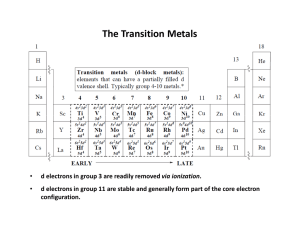
The Transition Metals
... orbitals oriented orthogonal wrt each other creating unique possibilities for ligand overlap. ...
... orbitals oriented orthogonal wrt each other creating unique possibilities for ligand overlap. ...
Ultra rigid cross-bridged tetraazamacrocycles as ligands—the
... ion and the Nax–M–Nax bond angle, which increases smoothly from MnII through CuII as the smaller metal ions can more easily be engulfed by the macrobicycle. MnII(5)Cl2 (Fig. 1) exemplifies these structures,§ and in this example the N(3)–Mn(1)–N(4) angle is 158.0°. Because of their great importance i ...
... ion and the Nax–M–Nax bond angle, which increases smoothly from MnII through CuII as the smaller metal ions can more easily be engulfed by the macrobicycle. MnII(5)Cl2 (Fig. 1) exemplifies these structures,§ and in this example the N(3)–Mn(1)–N(4) angle is 158.0°. Because of their great importance i ...
Phosphine Ligands
... If we consider the Schrock carbene as a Fischer carbene with strongly enhanced M→ →C p-back bonding, the 2 electrons originally in M(dπ) transfer to the C(pz) orbital, oxidizing the metal by 2 units and giving a CR22- ligand. ...
... If we consider the Schrock carbene as a Fischer carbene with strongly enhanced M→ →C p-back bonding, the 2 electrons originally in M(dπ) transfer to the C(pz) orbital, oxidizing the metal by 2 units and giving a CR22- ligand. ...
Preparation of Aldehydes and Ketones
... The carbocation-like carbon atom is stabilized by alkyl groups and destabilized by electron-withdrawing groups, such as CCl=3= and CF3. The stabilities of the product diols are affected to a lesser extent. ...
... The carbocation-like carbon atom is stabilized by alkyl groups and destabilized by electron-withdrawing groups, such as CCl=3= and CF3. The stabilities of the product diols are affected to a lesser extent. ...
ic100p12a
... (b) lead chromate: PbCrO4, yellow highway markings (paint). (c) sodium dichromate: Na2Cr2O7, for oxidation of organic compounds (e.g., alcohols to acids). (d) chromium(III) oxide: Cr2O3, green pigment, used in printing US currency (greenbacks). (e) molybdenum sulfide: MoS2, a layered compound used a ...
... (b) lead chromate: PbCrO4, yellow highway markings (paint). (c) sodium dichromate: Na2Cr2O7, for oxidation of organic compounds (e.g., alcohols to acids). (d) chromium(III) oxide: Cr2O3, green pigment, used in printing US currency (greenbacks). (e) molybdenum sulfide: MoS2, a layered compound used a ...
π bonded ligands
... The report in 1825 by William Zeise of crystals with composition, KCl.PtCl2.EtOH, prepared from KPtCl4 and EtOH was a topic of controversy for many years due to the nature of Zeise’s structure - only possible by the dehydration of EtOH. ...
... The report in 1825 by William Zeise of crystals with composition, KCl.PtCl2.EtOH, prepared from KPtCl4 and EtOH was a topic of controversy for many years due to the nature of Zeise’s structure - only possible by the dehydration of EtOH. ...
Metal Sequestration (English version)
... with a free concentration noticeably reduced, depending on the strength of the sequestering agent, i.e. on the stability of complex species formed by the metal ion with the ligand added or natural occurring in solution (such as, for example, the humic substance in natural waters). The reduction of f ...
... with a free concentration noticeably reduced, depending on the strength of the sequestering agent, i.e. on the stability of complex species formed by the metal ion with the ligand added or natural occurring in solution (such as, for example, the humic substance in natural waters). The reduction of f ...
Electronic spectrum of a 0.1 M aqueous solution of [Ti(H2O)6]3+
... Tetragonal distortions from octahedral symmetry (Jahn-Teller effect): - tetragonal distortions may occur when all 6 ligands of a complex (ML6) are the same (two of the ligands [in trans position] may be closer or further away from the metal centre) - the basis for this phenomenon is formulated in t ...
... Tetragonal distortions from octahedral symmetry (Jahn-Teller effect): - tetragonal distortions may occur when all 6 ligands of a complex (ML6) are the same (two of the ligands [in trans position] may be closer or further away from the metal centre) - the basis for this phenomenon is formulated in t ...
Redox & Complex Ion Reactions
... • Although the names of complex ions can look crazy, the formula are simply knowing the patterns, much like naming ...
... • Although the names of complex ions can look crazy, the formula are simply knowing the patterns, much like naming ...
Redox & Complex Ion Reactions
... • Although the names of complex ions can look crazy, the formula are simply knowing the patterns, much like naming ...
... • Although the names of complex ions can look crazy, the formula are simply knowing the patterns, much like naming ...
Activity 5 – Catalytic Cycles
... We will look at a number of catalytic cycles below, but first it is useful to think about the key steps which make up all the catalytic cycles. 1) Oxidative Addition (Figure 7). In this step the metal in the centre loses electrons to the ligands as the ligand are added to the metal centre, hence the ...
... We will look at a number of catalytic cycles below, but first it is useful to think about the key steps which make up all the catalytic cycles. 1) Oxidative Addition (Figure 7). In this step the metal in the centre loses electrons to the ligands as the ligand are added to the metal centre, hence the ...
The use of conductivity measurements in organic solvents for the
... England March 18, 1981 “For several years before 1970 my research group had been investigating transition metal complexes of heterocyclic ligands, particularly from the viewpoint of their possible use as analytical reagents. As so often happens, although this aspect proved to be disappointing anothe ...
... England March 18, 1981 “For several years before 1970 my research group had been investigating transition metal complexes of heterocyclic ligands, particularly from the viewpoint of their possible use as analytical reagents. As so often happens, although this aspect proved to be disappointing anothe ...
Trace Metal Biogeochemistry 12.755
... • Critical.exe – Smith and Martell volumes built into a DOS baseddatabase. • But need to know how to do it by hand well in order to use software effectively. I usually use both hand calculations and computer assisted calculations to cross-check assumptions. ...
... • Critical.exe – Smith and Martell volumes built into a DOS baseddatabase. • But need to know how to do it by hand well in order to use software effectively. I usually use both hand calculations and computer assisted calculations to cross-check assumptions. ...
CoordinationCompounds
... Coordination Compounds • Transition metals have s, d and p orbitals all available for bonding • Don’t obey the octet rule • They are most stable with filled d, s and p orbitals – s2d10p6 (18 e-) • Transition metals act like a Lewis acid (electron pair acceptor) so as to fill valence orbitals • Tran ...
... Coordination Compounds • Transition metals have s, d and p orbitals all available for bonding • Don’t obey the octet rule • They are most stable with filled d, s and p orbitals – s2d10p6 (18 e-) • Transition metals act like a Lewis acid (electron pair acceptor) so as to fill valence orbitals • Tran ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.











![Electronic spectrum of a 0.1 M aqueous solution of [Ti(H2O)6]3+](http://s1.studyres.com/store/data/005719667_1-a6d66a78471c6778162e27e0ef555131-300x300.png)









