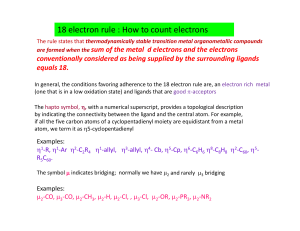
18 electron rule : How to count electrons
... comparatively lower energy which makes it possible to interact with metal t2g orbitals for π bonding. There exists a strong back bonding of metal electrons to the π * antibonding orbitals of CO ...
... comparatively lower energy which makes it possible to interact with metal t2g orbitals for π bonding. There exists a strong back bonding of metal electrons to the π * antibonding orbitals of CO ...
role of the ancillary ligands on the coordination modes
... DNA components with a metal centres deal with complexes of platinum(II) stabilized by N-donor ligands (NH3 and amines)[1]. Here we report the reactivity of the nucleobases 1-methylcytosine (1MeCy) and 9-methyladenine (9-MeAd) toward the hydroxo complexes cis-[L2Pt(µ-OH)]2(NO3)2, (L= PMe3, 1a; PMe2Ph ...
... DNA components with a metal centres deal with complexes of platinum(II) stabilized by N-donor ligands (NH3 and amines)[1]. Here we report the reactivity of the nucleobases 1-methylcytosine (1MeCy) and 9-methyladenine (9-MeAd) toward the hydroxo complexes cis-[L2Pt(µ-OH)]2(NO3)2, (L= PMe3, 1a; PMe2Ph ...
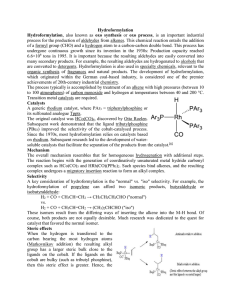
Hydroformylation Hydroformylation, also known as oxo synthesis or
... Thus, as a result, the electronic effects that favour the Markovnikov addition to an alkene are less able to direct the hydride to the carbon atom bearing the most hydrogens already. Thus, as a result, as the metal centre becomes more electron-rich, the catalyst becomes more selective for the straig ...
... Thus, as a result, the electronic effects that favour the Markovnikov addition to an alkene are less able to direct the hydride to the carbon atom bearing the most hydrogens already. Thus, as a result, as the metal centre becomes more electron-rich, the catalyst becomes more selective for the straig ...
Nugget
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
... Investigation of the Lewis Acidic Properties of N-Heterocyclic Carbene Ligands and Their Implications for Catalysis Colin D. Abernethy, Department of Chemistry, Keene State College Keene, NH 03435 Cyclopentadienyl complexes of vanadium chlorides, which also contain N-heterocyclic carbene (NHC) ligan ...
Medicinal Properties of Transition Metal Complexes
... effective for ER+ breast cancer.8 Another example involves Sadler and coworkers who discovered a ruthenium complex (Fig. 1e) that catalyzes the oxidation of glutathione (GSH) to glutathione dimers (GSSG), sensitizing cancer cells to ROS leading to an inhibition of growth.9 In both cases alteration o ...
... effective for ER+ breast cancer.8 Another example involves Sadler and coworkers who discovered a ruthenium complex (Fig. 1e) that catalyzes the oxidation of glutathione (GSH) to glutathione dimers (GSSG), sensitizing cancer cells to ROS leading to an inhibition of growth.9 In both cases alteration o ...
effective oxidation states applied to endohedral - IQCC
... electronic or spin populations are only a pointer of the atom's OS. We have most recently shown that the so-called effective atomic orbitals (eff-AO's) can be utilized, treating alpha and beta electrons separately, to derive the most appropriate atomic electron configurations for the atoms or molecu ...
... electronic or spin populations are only a pointer of the atom's OS. We have most recently shown that the so-called effective atomic orbitals (eff-AO's) can be utilized, treating alpha and beta electrons separately, to derive the most appropriate atomic electron configurations for the atoms or molecu ...
ncur_powerpoint Courtney
... Typical Metal Complex: [Ni3(tris-CB-Cyclens)(OAc)3](PF6)3 ∙ 6H2O Elemental Analysis Calculated as Ni3C48H87N12O6P3F18 ∙ 6 H2O: Calc: C 35.00, H 6.06, N 10.20; Found: C 34.66, H 5.61, N 10.20 ...
... Typical Metal Complex: [Ni3(tris-CB-Cyclens)(OAc)3](PF6)3 ∙ 6H2O Elemental Analysis Calculated as Ni3C48H87N12O6P3F18 ∙ 6 H2O: Calc: C 35.00, H 6.06, N 10.20; Found: C 34.66, H 5.61, N 10.20 ...
Departmental Seminar Candidate Graham de Ruiter
... terminal oxo ligands are common intermediates and consequently, many monometallic iron model complexes exhibiting terminal oxo ligands have been investigated. Despite the intense research efforts, terminal oxo motifs on a multi-metallic scaffold have not yet been reported. Recently we presented a ne ...
... terminal oxo ligands are common intermediates and consequently, many monometallic iron model complexes exhibiting terminal oxo ligands have been investigated. Despite the intense research efforts, terminal oxo motifs on a multi-metallic scaffold have not yet been reported. Recently we presented a ne ...
Team In Toulouse
... of olefins and the hydrosilylation of carbonyl groups. The immobilization of the novel catalysts in ionic liquids will be also explored in order to efficiently recover the homogenous catalysts. ...
... of olefins and the hydrosilylation of carbonyl groups. The immobilization of the novel catalysts in ionic liquids will be also explored in order to efficiently recover the homogenous catalysts. ...
ORGANOMETALLIC COMPOUNDS
... Organometallic compounds are compounds which contain bonds between carbon and a metal. To differentiate the compounds of these type, we use the nomenclature E‐C (E binds to carbon where E is a P‐block metalloid), and M‐C (M binds to carbon where M is an electropositive meta ...
... Organometallic compounds are compounds which contain bonds between carbon and a metal. To differentiate the compounds of these type, we use the nomenclature E‐C (E binds to carbon where E is a P‐block metalloid), and M‐C (M binds to carbon where M is an electropositive meta ...
Slide 1
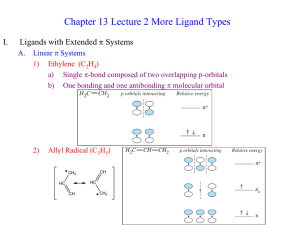
... 3) p-MO’s of cyclic conjugated ligands For qualitative analysis of bonding between transition metals and p-electron donors such as organic compounds with C=C bonds it is necessary to know how the ligand p-MO’s look like. Frost diagrams allow establish the shape, degeneracy and the energy sequence o ...
... 3) p-MO’s of cyclic conjugated ligands For qualitative analysis of bonding between transition metals and p-electron donors such as organic compounds with C=C bonds it is necessary to know how the ligand p-MO’s look like. Frost diagrams allow establish the shape, degeneracy and the energy sequence o ...
Transition Metal Carbonyls
... CO groups have a high tendency to stabilize M−M bonds; not only are CO ligands relatively small but they also leave the metal atom with a net charge similar to that in its elemental form (electroneutrality principle). “Stable complexes are those with structures such that each atom has only a small e ...
... CO groups have a high tendency to stabilize M−M bonds; not only are CO ligands relatively small but they also leave the metal atom with a net charge similar to that in its elemental form (electroneutrality principle). “Stable complexes are those with structures such that each atom has only a small e ...
Chapter 1 Structure and Bonding
... a) Single p-bond composed of two overlapping p-orbitals b) One bonding and one antibonding p molecular orbital ...
... a) Single p-bond composed of two overlapping p-orbitals b) One bonding and one antibonding p molecular orbital ...
Staff demonstrating hours for level-3 Inorganic Lab
... W(=CH2) 1.94 Å Difficult to separate effects of 3 components in metal complexes. Evidence for -donor orbital (cases where no -bonding is possible) Lewis adduct H3BCO. Complex has (CO) at 2164cm-1 , free CO at 2143cm-1 therefore C-O bond order is increased with donation, as predicted. Same f ...
... W(=CH2) 1.94 Å Difficult to separate effects of 3 components in metal complexes. Evidence for -donor orbital (cases where no -bonding is possible) Lewis adduct H3BCO. Complex has (CO) at 2164cm-1 , free CO at 2143cm-1 therefore C-O bond order is increased with donation, as predicted. Same f ...
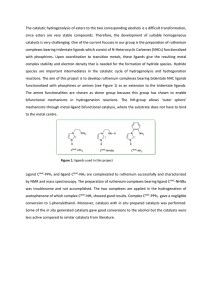
The catalytic hydrogenolysis of esters to the two corresponding
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
Coordination Chemistry
... terminal CO groups show absorption bands close to that of carbon monoxide [C-O stretching frequency of carbon monoxide = 2143 cm-1, terminal CO = 2125 to 2000 cm-1], while the bridging CO groups show absorption bands close to that of a keto group [1900 to 1800 cm-1]. But the possible existence of - ...
... terminal CO groups show absorption bands close to that of carbon monoxide [C-O stretching frequency of carbon monoxide = 2143 cm-1, terminal CO = 2125 to 2000 cm-1], while the bridging CO groups show absorption bands close to that of a keto group [1900 to 1800 cm-1]. But the possible existence of - ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
1. Acetone can bind to transition metals via oxygen only, or via both
... Low-valent, late transition metals that favor soft π-ligands should favor η2coordination. High-valent, early transition metals that favor hard ligands (such as oxygen ligands) should favor η1-coordination. Large ancillary ligands should favor η1-coordination. Both forms should promote nucleophilic a ...
... Low-valent, late transition metals that favor soft π-ligands should favor η2coordination. High-valent, early transition metals that favor hard ligands (such as oxygen ligands) should favor η1-coordination. Large ancillary ligands should favor η1-coordination. Both forms should promote nucleophilic a ...
Chemistry 332 Basic Inorganic Chemistry II
... Ni reacts with CO (leaving the impurities behind), to form Ni(CO)4. The Ni(CO)4 is passed through a tower filled with nickel pellets at a high velocity and 400 K. Pure Ni plates out on the pellets. * A commercial process for the manufacture of Na2CO3. NH3 and CO2 are passed into a sat’d NaCl(aq) sol ...
... Ni reacts with CO (leaving the impurities behind), to form Ni(CO)4. The Ni(CO)4 is passed through a tower filled with nickel pellets at a high velocity and 400 K. Pure Ni plates out on the pellets. * A commercial process for the manufacture of Na2CO3. NH3 and CO2 are passed into a sat’d NaCl(aq) sol ...
Abstract
... replacement of chloro ligands by azido ligands occurred, yielding a dimer with square planar configuration at each metal center. Abstraction of the chloro ligand from the monomer followed by reaction with sodium azide was also unsuccessful. However, reaction of the monomer with methyllithium led to ...
... replacement of chloro ligands by azido ligands occurred, yielding a dimer with square planar configuration at each metal center. Abstraction of the chloro ligand from the monomer followed by reaction with sodium azide was also unsuccessful. However, reaction of the monomer with methyllithium led to ...
Transition Metals
... The 3d orbitals are not as important for bonding as are the 4s and 4p, but the details of what happens to the 3d orbitals determine the properties of transition metal complexes. ...
... The 3d orbitals are not as important for bonding as are the 4s and 4p, but the details of what happens to the 3d orbitals determine the properties of transition metal complexes. ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.