Organometallics
... order [Ar]4s23d10, this turns out to be true only for isolated metal atoms. When we put a metal ion into an electronic field (surround it with ligands), the d-orbitals drop in energy and fill first. Therefore, the organometallic chemist considers the transition metal valence electrons to all be d-el ...
... order [Ar]4s23d10, this turns out to be true only for isolated metal atoms. When we put a metal ion into an electronic field (surround it with ligands), the d-orbitals drop in energy and fill first. Therefore, the organometallic chemist considers the transition metal valence electrons to all be d-el ...
Intro to Transition Metal Complexes(CH 21) Valence Bond Theory
... described as the combination of a metal cation and a group of Lewis base ligands (usually 4 or 6, though many other numbers are known), these compounds of formula ML n : • Are often colored. Color is a rare phenomenon at the chemical compound level. Most compounds are colorless. • the color will var ...
... described as the combination of a metal cation and a group of Lewis base ligands (usually 4 or 6, though many other numbers are known), these compounds of formula ML n : • Are often colored. Color is a rare phenomenon at the chemical compound level. Most compounds are colorless. • the color will var ...
A1988Q406500001
... ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occurred to me then diminished in quantity, has largely been more that by studying complexes of molecul ...
... ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occurred to me then diminished in quantity, has largely been more that by studying complexes of molecul ...
A1988Q406700001
... ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occurred to me then diminished in quantity, has largely been more that by studying complexes of molecul ...
... ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occurred to me then diminished in quantity, has largely been more that by studying complexes of molecul ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 24. a) Explain the features of Tanabe- Sugano and Orgel diagrams. b) Construct Orgel diagram for d3and d7 systems for tetrahedral and octahedral complexes. (5+5) 25. a) Explain why is the stretching frequency of νC - N shifted to higher ν while that of νC - O is lowered on back donation from metal t ...
... 24. a) Explain the features of Tanabe- Sugano and Orgel diagrams. b) Construct Orgel diagram for d3and d7 systems for tetrahedral and octahedral complexes. (5+5) 25. a) Explain why is the stretching frequency of νC - N shifted to higher ν while that of νC - O is lowered on back donation from metal t ...
Experimental study on transition metal complexes containing N,S
... complexes along with [Mo(tLNS)3] (11) have been studied and it has been found that the oxidation state of +5 is present for the central metal ion for all these compounds. In contrast to the previous literature reports viewing the neutral complexes as MVI (d0) configuration, we have found that the ne ...
... complexes along with [Mo(tLNS)3] (11) have been studied and it has been found that the oxidation state of +5 is present for the central metal ion for all these compounds. In contrast to the previous literature reports viewing the neutral complexes as MVI (d0) configuration, we have found that the ne ...
Chemistry 332 Basic Inorganic Chemistry II
... valence orbitals and thus such compounds obey this rule. ...
... valence orbitals and thus such compounds obey this rule. ...
ELECTRON COUNTING IN TRANSITION METAL COMPLEXES
... charge. As a result, it does not have ten electrons. It only has eight. The total electron count on the metal in the complex is sixteen. ...
... charge. As a result, it does not have ten electrons. It only has eight. The total electron count on the metal in the complex is sixteen. ...
Chapter 1 Structure and Bonding
... a) Each bond between metals counts 1 electron per metal: Mn—Mn = 1 eb) Total ligand charge = 0, so Mn0 = d7 c) 5 CO ligands per metal = 10 electrons for a total of 18 electrons per Mn ...
... a) Each bond between metals counts 1 electron per metal: Mn—Mn = 1 eb) Total ligand charge = 0, so Mn0 = d7 c) 5 CO ligands per metal = 10 electrons for a total of 18 electrons per Mn ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
Homework 1 - IONiC / VIPEr
... oxidation state, dn count, and total valence electron count. While a useful formalism, you should be aware that an isolated Cr+6 ion is really not present in a complex ion. For this reason, metal ions are usually written with roman numerals like Cr(VI). The oxidation state is the formal charge on th ...
... oxidation state, dn count, and total valence electron count. While a useful formalism, you should be aware that an isolated Cr+6 ion is really not present in a complex ion. For this reason, metal ions are usually written with roman numerals like Cr(VI). The oxidation state is the formal charge on th ...
TRANSITION METALS - Pennsylvania State University
... Occupy the d-block of periodic table Have d-electrons in valence shell ...
... Occupy the d-block of periodic table Have d-electrons in valence shell ...
Organometallic Chemistry
... Chain-end control mechanism: the polymer chain determines the stereospecificity of the final polymer. ...
... Chain-end control mechanism: the polymer chain determines the stereospecificity of the final polymer. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... Answer all questions. Each question carries two t marks. ...
... Answer all questions. Each question carries two t marks. ...
Ch 24 Part 2 PowerPoint
... ligands along the z-axis removed. As a consequence the four planar ligands are drawn in closer towards the metal. Relative to the octahedral field, the dz2 orbital is greatly lowered in energy, the dyz, and dxz orbitals lowered in energy, the dxy, and dx2-y2 orbitals are raised in energy. ...
... ligands along the z-axis removed. As a consequence the four planar ligands are drawn in closer towards the metal. Relative to the octahedral field, the dz2 orbital is greatly lowered in energy, the dyz, and dxz orbitals lowered in energy, the dxy, and dx2-y2 orbitals are raised in energy. ...
Into to metal complexes
... Ligands are species (neutral or anionic) bonded to the metal ion They may be attached to the metal through a single atom (monodentate) or bound to the metal through two or more atoms (bidentate, tridentate etc.) Polydentate ligands are called chelating ligands ...
... Ligands are species (neutral or anionic) bonded to the metal ion They may be attached to the metal through a single atom (monodentate) or bound to the metal through two or more atoms (bidentate, tridentate etc.) Polydentate ligands are called chelating ligands ...
Redox Reactions
... 3) Addition of H-, H+ – hydride reagents. Hydride reactions are nucleophilic reactions that reduce polar compounds. They are typically followed by an aqueous or acidic work-up that provides the proton. Addition of H2 These reductions rely on transition metal catalysts to split a molecule of H2 and t ...
... 3) Addition of H-, H+ – hydride reagents. Hydride reactions are nucleophilic reactions that reduce polar compounds. They are typically followed by an aqueous or acidic work-up that provides the proton. Addition of H2 These reductions rely on transition metal catalysts to split a molecule of H2 and t ...
H,mR,cn,nii,f^{aq) + H,O(1)
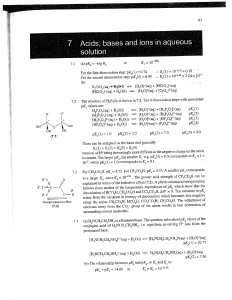
... Class (b), or soft, metal ions prefer soft donor atoms, e.g. S rather than O, and P rather than N. Of the metal ions given in the question, Gd^^ ajid_ Fe^-^ are hard aeids while Ag"^ is a soft metal ion. The pattern in values of log JTis consistent with a favourable hard metal ion-hard ligand match, ...
... Class (b), or soft, metal ions prefer soft donor atoms, e.g. S rather than O, and P rather than N. Of the metal ions given in the question, Gd^^ ajid_ Fe^-^ are hard aeids while Ag"^ is a soft metal ion. The pattern in values of log JTis consistent with a favourable hard metal ion-hard ligand match, ...
Organometallic Reactions and Catalysis
... • Organometallic complexes can behave as nucleophiles in displacement reactions (especially negativelycharged complexes). [Co(CO)4]- + RX RCo(CO)4 + XRCo(CO)4 + CO R(C=O)Co(CO)4 (acyl complex) R(C=O)Co(CO)4 +R’OH R(C=O)OR’ + HCo(CO)4 (generates the ester from an alcohol). ...
... • Organometallic complexes can behave as nucleophiles in displacement reactions (especially negativelycharged complexes). [Co(CO)4]- + RX RCo(CO)4 + XRCo(CO)4 + CO R(C=O)Co(CO)4 (acyl complex) R(C=O)Co(CO)4 +R’OH R(C=O)OR’ + HCo(CO)4 (generates the ester from an alcohol). ...
Click
... by combination of CO molecules, with transition metals are known as metallic carbonyls. Since the electrons supplied solely by CO molecule in the formation of OC→M bond, metal atom in carbonyl has zero oxidation state. ...
... by combination of CO molecules, with transition metals are known as metallic carbonyls. Since the electrons supplied solely by CO molecule in the formation of OC→M bond, metal atom in carbonyl has zero oxidation state. ...
OMC-Karlin-Spring-`09-Lecture-Set
... equatorial to axial positions. Ligand 1 does not move and acts as a pivot. At the midway point (transition state) ligands 2,3,4,5 are equivalent, forming the base of a square pyramid. The motion is equivalent to a 90° rotation about the M-L1 axis. Molecular examples could be PF5 or Fe(CO)5. ...
... equatorial to axial positions. Ligand 1 does not move and acts as a pivot. At the midway point (transition state) ligands 2,3,4,5 are equivalent, forming the base of a square pyramid. The motion is equivalent to a 90° rotation about the M-L1 axis. Molecular examples could be PF5 or Fe(CO)5. ...
Modelling for Coordination Complexes: Structure
... unsophisticated compared to QM, MM can be a remarkably good way of modelling molecules, especially those based on carbon chemistry, because carbon behaves in clear, well-defined ways which are relatively simple to capture mathematically. For example, each carbon atom is connected to four, three or t ...
... unsophisticated compared to QM, MM can be a remarkably good way of modelling molecules, especially those based on carbon chemistry, because carbon behaves in clear, well-defined ways which are relatively simple to capture mathematically. For example, each carbon atom is connected to four, three or t ...
Coordination Compounds
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
... • Complex ion: can be cation or anion; contains the transition metal and usually is in brackets. • Ligands: neutral molecule or ion having a lone pair of electrons that forms a coordinate covalent bond to a metal ion; usually dissociate when aqueous. Ligands in the spectrochemical series show which ...
Transition Metal Chemistry
... Multiple oxidation states attainable via ionization energies. In order to have higher oxidation states, there needs to be greater packets of (single) energy. Note: removal of successive e-'s ...
... Multiple oxidation states attainable via ionization energies. In order to have higher oxidation states, there needs to be greater packets of (single) energy. Note: removal of successive e-'s ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.