Chem 174_Lecture 10a..
... • The metal has to exhibit a low oxidation state in order for these complexes to be stable • The s-bond is formed from the sp-orbital of the carbon atom with a suitable empty d-orbital of the metal while the p-back bond is formed by the interaction of a filled d-orbital of the metal with the p*-orbi ...
... • The metal has to exhibit a low oxidation state in order for these complexes to be stable • The s-bond is formed from the sp-orbital of the carbon atom with a suitable empty d-orbital of the metal while the p-back bond is formed by the interaction of a filled d-orbital of the metal with the p*-orbi ...
Essentials of Coordination Chemistry Brochure
... centers, before discussing the variety of isomerism exhibited by coordination compounds, such as structural, geometrical and optical isomerism. As thermodynamics and kinetics provide a gateway to synthesis and reactivity of coordination compounds, the book then describes the determination of stabili ...
... centers, before discussing the variety of isomerism exhibited by coordination compounds, such as structural, geometrical and optical isomerism. As thermodynamics and kinetics provide a gateway to synthesis and reactivity of coordination compounds, the book then describes the determination of stabili ...
What is ORGANOMETALLIC Chemistry
... simple interpretation of UV-Vis spectra and magnetic moment measurements. (8) Ligands less labile for 3rd row TM complexes compared with 2 nd row TM complexes – 2nd row TM complexes often best for catalysis. ...
... simple interpretation of UV-Vis spectra and magnetic moment measurements. (8) Ligands less labile for 3rd row TM complexes compared with 2 nd row TM complexes – 2nd row TM complexes often best for catalysis. ...
Staff demonstrating hours for level-3 Inorganic Lab
... KReO4 + K/EtOH "K2Re" white crystalline solid Modern techniques X-ray, NMR not available. Compound actually contains the anion [ReH9]2- - an 18 electron complex !! ...
... KReO4 + K/EtOH "K2Re" white crystalline solid Modern techniques X-ray, NMR not available. Compound actually contains the anion [ReH9]2- - an 18 electron complex !! ...
Scientific abstract
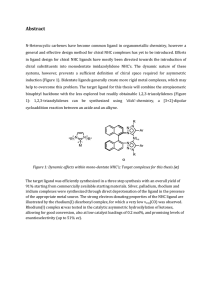
... 1): 1,2,3-triazolylidenes can be synthesized using ‘click’-chemistry, a [3+2]-dipolar cycloaddition reaction between an azide and an alkyne. ...
... 1): 1,2,3-triazolylidenes can be synthesized using ‘click’-chemistry, a [3+2]-dipolar cycloaddition reaction between an azide and an alkyne. ...
1 5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 27 April 11
... Why? As we have now shown with AOM and MO methods, different ligands engender different values of ∆O. The field strength of various ligands may be assessed by measuring ∆O for different ligands about a given metal ion of given charge, ...
... Why? As we have now shown with AOM and MO methods, different ligands engender different values of ∆O. The field strength of various ligands may be assessed by measuring ∆O for different ligands about a given metal ion of given charge, ...
Synthesis of hydroborate compounds as potential chemical vapor
... complexes of d-block transition metals are rare because the BH4- ligand is sterically small and strongly reducing, and in fact only Ti(BH4)3, Zr(BH4)4, and Hf(BH4)4 are known.8,9 By employing the sterically more demanding hydroborate ligand B3H8-, the highly volatile chromium(II) complex of Cr(B3H8) ...
... complexes of d-block transition metals are rare because the BH4- ligand is sterically small and strongly reducing, and in fact only Ti(BH4)3, Zr(BH4)4, and Hf(BH4)4 are known.8,9 By employing the sterically more demanding hydroborate ligand B3H8-, the highly volatile chromium(II) complex of Cr(B3H8) ...
Inorganic Chemistry review sheet Exam #3 Ch. 9 Lewis acids (e
... Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy." Can influence symmetry (will see one point group via crystallography instead of ano ...
... Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy." Can influence symmetry (will see one point group via crystallography instead of ano ...
Key Concepts PowerPoint
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
Electron Counting, Formal Oxidation States and the 18 Electron
... d shells for a total of 18 electrons in the 9 valence orbitals, he reasoned that metal complexes with 18 electrons might also exhibit particularly high stability. This "18 electron rule" (also called the effective atomic number rule) is analogous to the octet rule discussed in earlier courses and is ...
... d shells for a total of 18 electrons in the 9 valence orbitals, he reasoned that metal complexes with 18 electrons might also exhibit particularly high stability. This "18 electron rule" (also called the effective atomic number rule) is analogous to the octet rule discussed in earlier courses and is ...
Crystal Field Theory www.AssignmentPoint.com Crystal Field
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
Chem 30CL-Lecture 15..
... Cp-ring with electron-donating groups that raise the energy of the anti-bonding orbitals i.e., Co(CpMe5)2: (E0= -1.94 V vs. FeCp2) Placing electron-accepting groups on the Cp-ring makes the reduction potential more positive i.e., acetylferrocene E0= 0.24 V vs. FeCp2), cyanoferrocene (E0= 0.36 V vs ...
... Cp-ring with electron-donating groups that raise the energy of the anti-bonding orbitals i.e., Co(CpMe5)2: (E0= -1.94 V vs. FeCp2) Placing electron-accepting groups on the Cp-ring makes the reduction potential more positive i.e., acetylferrocene E0= 0.24 V vs. FeCp2), cyanoferrocene (E0= 0.36 V vs ...
Lecture 14a - University of California, Los Angeles
... • Cobaltocene is a strong reducing reagent (E0= -1.33 V vs. FeCp2) because it is a 19 valence electron system with its highest electron in an anti-bonding orbital • The oxidation with iodine leads to the light-green cobaltocenium ion • It is often used as counter ion to crystallize large anions (158 ...
... • Cobaltocene is a strong reducing reagent (E0= -1.33 V vs. FeCp2) because it is a 19 valence electron system with its highest electron in an anti-bonding orbital • The oxidation with iodine leads to the light-green cobaltocenium ion • It is often used as counter ion to crystallize large anions (158 ...
08_ synopsis
... themselves the test subject in many phases of modern chemical scientific research and development. Metal carbonyls are coordination complexes of transition metals with carbon monoxide. These complexes may be homoletic, i.e. contain only CO ligands, such as nickel carbonyl (Ni(CO)4), but more commonl ...
... themselves the test subject in many phases of modern chemical scientific research and development. Metal carbonyls are coordination complexes of transition metals with carbon monoxide. These complexes may be homoletic, i.e. contain only CO ligands, such as nickel carbonyl (Ni(CO)4), but more commonl ...
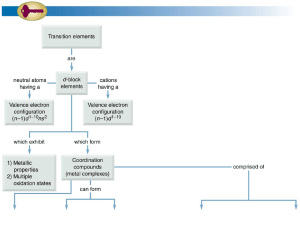
Transition Metals introduction
... At A Level, you only need to know about: Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni) and Copper (Cu) ...
... At A Level, you only need to know about: Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni) and Copper (Cu) ...
Organometallic Compounds
... • Discuss the proton NMR of Cr(CO)5[C(OCH3)C6H5]. • At high temperatures there is one signal from the methyl protons and at low temperatures there is one signal. Why? ...
... • Discuss the proton NMR of Cr(CO)5[C(OCH3)C6H5]. • At high temperatures there is one signal from the methyl protons and at low temperatures there is one signal. Why? ...
5_slides_olefin_complexes_VIPEr
... Created by Margaret L. Scheuermann, Princeton University; Abby R. O’Connor, The College of New Jersey, [email protected]. Copyright Scheuermann and O’Connor, 2014. This work is licensed under the Creative Commons Attribution-NonCommercialShareAlike 3.0 Unported License. To view a copy of this licens ...
... Created by Margaret L. Scheuermann, Princeton University; Abby R. O’Connor, The College of New Jersey, [email protected]. Copyright Scheuermann and O’Connor, 2014. This work is licensed under the Creative Commons Attribution-NonCommercialShareAlike 3.0 Unported License. To view a copy of this licens ...
Organometallic Chemistry
... donate electrons to the π–orbitals of the CO Thus, metal ions with higher formal charges, e.g. Fe(II) form CO complexes with much greater difficulty than do zero-valent metal ions For example Cr(O) and Ni(O), or negatively charged metal ions such as V(-I) ...
... donate electrons to the π–orbitals of the CO Thus, metal ions with higher formal charges, e.g. Fe(II) form CO complexes with much greater difficulty than do zero-valent metal ions For example Cr(O) and Ni(O), or negatively charged metal ions such as V(-I) ...
Document
... donate electrons to the π–orbitals of the CO Thus, metal ions with higher formal charges, e.g. Fe(II) form CO complexes with much greater difficulty than do zero-valent metal ions For example Cr(O) and Ni(O), or negatively charged metal ions such as V(-I) ...
... donate electrons to the π–orbitals of the CO Thus, metal ions with higher formal charges, e.g. Fe(II) form CO complexes with much greater difficulty than do zero-valent metal ions For example Cr(O) and Ni(O), or negatively charged metal ions such as V(-I) ...
Coordination Chemistry
... but are dependent on the choice of metal and ligand (in some cases the geometry of the complex can even change depending on variations in variables like temperature). There are many trends that correlate coordination number and geometry with the type of metal and ligand, but explaining these trends ...
... but are dependent on the choice of metal and ligand (in some cases the geometry of the complex can even change depending on variations in variables like temperature). There are many trends that correlate coordination number and geometry with the type of metal and ligand, but explaining these trends ...
Chapter 1 Structure and Bonding
... normally too high to allow the electron to pass through. This is a quantum mechanical process having to do with the wave nature of e-. ii. Ligands with p or p orbitals good for bonding more easily allow tunnelling (CN-, F-) than those that don’t (NH3). ...
... normally too high to allow the electron to pass through. This is a quantum mechanical process having to do with the wave nature of e-. ii. Ligands with p or p orbitals good for bonding more easily allow tunnelling (CN-, F-) than those that don’t (NH3). ...
InorgCh425
... The rates of reaction depend on the ability of the electron to “tunnel” through the ligands from one metal to the other i. Tunneling = moving through an energy barrier (the ligands) that is normally too high to allow the electron to pass through. This is a quantum mechanical process having to do wit ...
... The rates of reaction depend on the ability of the electron to “tunnel” through the ligands from one metal to the other i. Tunneling = moving through an energy barrier (the ligands) that is normally too high to allow the electron to pass through. This is a quantum mechanical process having to do wit ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 Part-A
... Answer any eight questions. Each question carries five marks. ...
... Answer any eight questions. Each question carries five marks. ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.