* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organometallic Chemistry
Ring-closing metathesis wikipedia , lookup
Aromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Marcus theory wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Cluster chemistry wikipedia , lookup
Stille reaction wikipedia , lookup
CHEM310
INORGANIC CHEMISTRY
Part 3
ORGANOMETALLIC CHEMISTRY
1. Introduction (types and rationale)
2. Molecular orbital (bonding) of CO, arrangement “in space”
or ligand types (hapticity)
3. 16 and 18 electron rule (learning to count)
4. Synthesis, steric effects and reactivity - Wilkinsons catalyst (part 1)
5. Characterisation IR nmr etc.
6. Applications (oxidative addition b elimination)
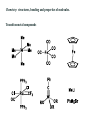
What is organometallic chemistry?
Chemistry: structures, bonding and properties of molecules.
Organometallic compounds: containing direct metal-carbon bonds.
Either s or p bonds can occur
Main group:
(AlMe3)2
Structures
s bonds and 3c-2e (or even 4c-2e) bonds
Chem 210
Synthesis
the first M-C bond
Reactivity
nucleophilic and basic
auxiliaries in organic synthesis
source of organic groups for transition metals
Strong preference for s-donor groups but Cp is often p-bound
(deceptively like with transition metals)
Cp2Mg
Cp2Fe
Electropositive metals: often 3c-2e or 4c-2e hydrides/alkyls
(Me3Al)2
(MeLi)4
As a Nucleophile
Addition to polar C=X bonds
(C=O, C=N, CºN)
R M
R
+
O
OM
Substitution at sp2 carbon
(often via addition)
R M
+
O
OR'
R
OR'
R
- MOR'
OM
O
Substitution at sp3 carbon does occur
but is far less easy and often has a multistep mechanism
Substitution at other elements:
often easy for polar M-X bonds
(Si-Cl, B-OMe)
Me
MeMgBr + B(OMe)3
BrMg
B(OMe)2
OMe
MeOMgBr + MeB(OMe)2
Me3B
As a base
More prominent in polar solvents think of free R- acting as base
Elimination
H
+ RH + MX
R M
X
mechanism can be more complex than this
Metallation
H
R M
Me2N
M
+ RH
Me2N
chelate effect more important than inductive effect!
b-hydrogen transfer
Al
H
O
Al
O
H
mainly for Al:
for more electropositive elements, deprotonation
and nucleophilic attack
are faster
for less electropositive elements, often no reaction
Chemistry: structures, bonding and properties of molecules.
Transition metal compounds
Some compounds do not contain metal-carbon bond, but they are usually
included in the field of organometallic chemistry. They include:
• Metal hydride complexes, e.g.
• Phosphine complexes, e.g.
• N2-complexes, e.g.
Exercise. Which of the following compounds is an organometallic compound?
NH 3
OCH 3
a)
Ti
CH3O
b)
Cl
H3 N
OCH 3
OCH 3
Pt
O
CH2
d)
O
CH2
Li
Li
O
Ph
Me
OC
Me
Pt
O
Cl
e)
NH 3
NH 3
In general, metals in
organometallic
compounds include:
-
Cl
c)
Cu
2+
Li
Me
Me
Li
P
CO
CO
O
Co C
Co CO
CO
f) OC Co
O C Co
OC
OC P
Ph
• main group metals
• transition metals
• f-block metals
In this course,
transition metals are
our main concern.
A brief history of organometallic chemistry
1) Organometallic Chemistry
has really been around for
millions of years
Naturally occurring
Cobalimins contain
Co—C bonds
Vitamin B12
2) Zeise’s Salt synthesized in 1827 = K[Pt(C2H4)Cl3] • H2O
Confirmed to have H2C=CH2 as a ligand in 1868
Structure not fully known until 1975
3) Ni(CO)4 synthesized in 1890
4) Grignard Reagents (XMgR) synthesized about
1900
Accidentally produced while trying to make other
compounds
Utility to Organic Synthesis recognized early on
5) Ferrocene synthesized in 1951
Modern Organometallic
Chemistry begins with this
discovery (Paulson and Miller)
1952 Fischer and Wilkinson
Nobel -Prize Winners related to the area:
Victor Grignard and Paul Sabatier (1912)
Grignard reagent
K. Ziegler, G. Natta (1963)
Zieglar-Natta catalyst
E. O. Fisher, G. Wilkinson (1973)
Sandwich compounds
K. B. Sharpless, R. Noyori (2001)
Hydrogenation and oxidation
Yves Chauvin, Robert H. Grubbs, Richard R. Schrock (2005) Metalcatalyzed alkene metathesis
Common organometallic ligands
M
H
M
M
M
M
CO
CS
C
M
M
M
M
C
CNR
NO
M
M
M
M
M
C
N2
PR3
M
M
H
H
M
M
H
X
M
M
C
M
C
Why organometallic chemistry ?
a). From practical point of view:
* OMC are useful for chemical synthesis, especially catalytic processes,
e.g. In production of fine chemicals
In production of chemicals in large-scale
reactions could not be achieved traditionally
* Organometallic chemistry is related to material sciences.
e.g. Organometallic Polymers
PBu3
Pt
C C
PBu3
C C
PBu3
n
Pt
PBu3
Small organometallic compounds:
Precursors to films for coating (MOCVD)
(h3-C3H5)2Pd -----> Pd film
CH3CC-Au-CNMe -----> Au film
Luminescent materials
C C
C C
n
* Biological Science. Organometallic chemistry may help us to
understand some enzyme-catalyzed reactions.
e.g. B12 catalyzed reactions.
H
R
R
H
b). From academic point of view:
* Organometallic compounds display many unexpected behaviorsdiscover new chemistry- new structures e.g.
H3N:
H
M
M
M
M
C
M
C
C
C
M
M
C
C
New reactions, reagents, catalysts, e.g.
Ziegler-Natta catalyst, Wilkinson catalyst
Reppe reaction, Schwartz's reagent
Sharpless epoxidation, Tebbe's reagent
M
H
H
M
SiR3
Types of bonds possible from Ligands
Language: All bonds are coordination or coordinative
Remember that all of these bonds are weaker than normal organic
bonds (they are dative bonds)
Simple ligands e.g. CH3-, Cl-, H2 give s bonds
systems are different e.g. CO is a s donor and p acceptor
Bridging ligands can occur two metals
Metal-metal bonds occur and are called d bonds – they are weak
and are a result of d-d orbital overlap
18 Electron Rule (Sidgwick, 1927)
• OM chemistry gives rise to many “stable” complexes - how can we
tell by a simple method
• Every element has a certain number of valence orbitals:
1 { 1s } for H
4 { ns, 3´np } for main group elements
9 { ns, 3´np, 5´(n-1)d } for transition metals
s
dxy
px
dxz
py
dyz
pz
dx2-y2
dz2
• Therefore, every element wants to be surrounded
by 2/8/18 electrons
– For main-group metals (8-e), this leads to the standard Lewis structure
rules
– For transition metals, we get the 18-electron rule
• Structures which have this preferred count are called
electron-precise
• Every orbital wants to be “used", i.e. contribute to binding an electron pair
The strength of the preference for electron-precise structures depends on the
position of the element in the periodic table
• For early transition metals, 18-e is often unattainable for steric reasons - the
required number of ligands would not fit
• For later transition metals, 16-e is often quite stable (square-planar d8
complexes)
• Addition of 2e- from 5th ligand converts complex to 5 CN 18e- , marginally
more stable
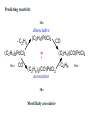
Predicting reactivity
14 e
- C2H4
(C2H4)2PdCl2
16 e
CO
dissociative
(C2H4)PdCl2
CO
?
(C2H4)2(CO)PdCl2
associative
18 e
Most likely associative
(C2H4)(CO)PdCl2
- C2H4
16 e
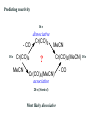
Predicting reactivity
16 e
- CO
18 e
Cr(CO)6
MeCN
dissociative
Cr(CO)5
MeCN
?
Cr(CO)5(MeCN) 18 e
Cr(CO)6(MeCN)
associative
20 e (Sterics!)
Most likely dissociative
- CO
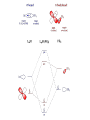
N.B. How do you know a fragment forms a covalent or a dative bond?
•
•
•
Chemists are "sloppy" in writing structures. A "line" can mean a
covalent bond, a dative bond, recognise/understand the bonding
first
Use analogies ("PPh3 is similar to NH3").
Rewrite the structure properly before you start counting.
Cl
PPh3
Cl
Pd
covalent
bond
1e
PPh3
2e
Pd
dative
bond
"bond" to the
allyl fragment
3e
Pd =
Cl¾ =
P® =
allyl =
10
1
2
3
+ ¾¾
e-count
16
"Covalent" count: (ionic method also useful)
1. Number of valence electrons of central atom.
• from periodic table
2. Correct for charge, if any
• but only if the charge belongs to that atom!
3. Count 1 e for every covalent bond to another atom.
4. Count 2 e for every dative bond from another atom.
• no electrons for dative bonds to another atom!
5. Delocalized carbon fragments: usually 1 e per C (hapticity)
6. Three- and four-center bonds need special treatment
7. Add everything
N.B. Covalent Model:
18 = (# metal electrons + # ligand electrons) - complex charge
The number of metal electrons equals it's row number (i.e., Ti = 4e, Cr = 6 e,
Ni = 10 e)
Hapto (h) Number (hapticity)
For some molecules the molecular formula provides insufficient
information with which to classify the metal carbon interactions
The hapto number (h) gives the number of carbon (conjugated) atoms
bound to the metal
It normally, but not necessarily, gives the number of electrons contributed
by the ligand
We will describe to methods of counting electrons but we will
employ only one for the duration of this module
The two methods compared:
some examples
N.B. like oxidation state
assignments, electron
counting is a formalism and does
not necessarily reflect the
distribution of electrons in the
molecule – useful though
Some ligands donate the same
number of electrons
Number of d-electrons and
donation of the other ligands
can differ
Now we will look at practical
examples on the black board
Does it look reasonable ?
Remember when counting:
Odd electron counts are rare
In reactions you nearly always go from even to even (or
odd to odd), and from n to n-2, n or n+2.
Electrons don’t just “appear” or “disappear”
The optimal count is 2/8/18 e. 16-e also occurs
frequently, other counts are much more rare.
Exceptions to the 18 Electron Rule
ZrCl2(C5H5)2 Zr(4) + [2 x Cl(1)] + [2 x C5H5(5)] =16
TaCl2Me3 Ta(5) + [2+ x Cl(1)] + [3 x M(1)] =10
WMe6 W(6) + [6 x Me(1)] =12
Pt(PPh3)3 Pt(10) + [3 x PPh3(2)] =16
IrCl(CO)(PPh3)2 Ir(9) + Cl(1) + CO(2) + [2 x PPh3(2)] =16
What features do these complexes possess?
• Early transition metals (Zr, Ta, W)
• Several bulky ligands (PPh3)
• Square planar d8 e.g. Pt(II), Ir(I)
• σ-donor ligands (Me)
Alkyl ligands:
Transition metal alkyl complexes important for catalysts e.g. olefin
polymerization and hydroformylation thermodynamic
Problem is their weak kinetic stability
(Thermally fine: M-C bond dissociation energies are typically 40-60
kcal/mol with 20-70 kcal/mol)
Simple alkyls are sigma donors, that can be considered to donate one or two
electrons to the metal center depending on which electron counting formalism
you use
Synthesis of Metal Alkyl Complexes
1. Metathetical exchange using a carbon nucleophile (R-). Common
reagents are RLi, RMgX (or R2Mg), ZnR2, AlR3, BR3, and PbR4. Much
of this alkylation chemistry can be understood with Pearson's "hardsoft" principles
2. Metal-centered nucleophiles (i.e. using R+ as a reagent) Typical
examples are a metal anion and alkyl halide (or pseudohalogen). for
example:
NaFp + RX
Fp-R + NaX
[Fp = Cp(CO)2Fe]
3. Oxidative Addition. This requires a covalently unsaturated, lowvalent complex (16 e- or less). A classic example:
4. Insertion- To form an alkyl, this usually involves an olefin
insertion. The simplest generic example is the insertion of ethylene
into an M-X bond, i.e.
M-X + CH2CH2
M-CH2CH2-X
Carbonyl Complexes
Bonding of CO
Electron donation of the lone pair
on carbon s This electron
donation makes the metal more
electron rich - compensate for this
increased electron density, a filled
metal d-orbital may interact with
the empty p* orbital on the
carbonyl ligand
p-backbonding or pbackdonation or synergistic
bonding
Similar for alkenes, acetylenes,
phosphines, and dihydrogen.
What stabilizes CO complexes is M→C π–bonding
The lower the formal charge on the metal ion the more willing it is to
donate electrons to the π–orbitals of the CO
Thus, metal ions with higher formal charges, e.g. Fe(II) form CO
complexes with much greater difficulty than do zero-valent metal ions
For example Cr(O) and Ni(O), or negatively charged metal ions such
as V(-I)
In general to get a feeling for stability examine the charges on the
metals
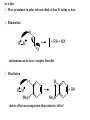
Syntheses of metal carbonyls
Metal carbonyls can be made in a variety of ways.
For Ni and Fe, the homoleptic or binary metal carbonyls can be made by the
direct interaction with the metal (Equation 1).
In other cases, a reduction of a metal precursor in the presence of CO (or using
CO as the reductant) is used (Equations 2-3).
Carbon monoxide also reacts with various metal complexes, most typically filling
a vacant coordination site (Equation 4) or performing a ligand substitution
reactions (Equation 5)
Occasionally, CO ligands are derived from the reaction of a coordinated ligand
through a deinsertion reaction (Equation 6)
Synthesis of carbonyl complexes
Direct reaction of the metal
– Not practical for all metals due to need for harsh
conditions (high P and T)
– Ni + 4CO Ni(CO)4
– Fe + 5CO Fe(CO)5
Reductive carbonylation
– Useful when very aggressive conditions would be
required for direct reaction of metal and CO
» Wide variety of reducing agents can be used
– CrCl3+ Al + 6CO AlCl3 + Cr(CO)6
– 3Ru(acac)3 + H2 + 12CO Ru3(CO)12 +
N.B. From the carbonyl complex we can synthesize other derivatives
Main characterization methods:
• Xray diffraction Þ (static) structure Þ bonding
• NMR Þ structure en dynamic behaviour
• EA Þ assessment of purity
• (calculations)
Useful on occasion:
• IR
• MS
• EPR
Not used much:
• GC
• LC
IR spectra and metal-carbon bonds
The υCO stretching frequency of the coordinated CO is very
informative
Recall that the stronger a bond gets, the higher its stretching
frequency
M=C=O (C=O is a double bond) canonical structure
Lower the υCO stretching frequency as compared to the M-C≡O
structure (triple bond)
Note: υCO for free CO is 2041 cm-1)
[Ti(CO)6]2- [V(CO)6]- [Cr(CO)6] [Mn(CO)6]+ [Fe(CO)6]2+
υCO
1748
1858
1984
increasing M=C double
bonding
2094
2204 cm-1
decreasing M=C double
bonding
Bridging versus terminal carbonyls
Bridging CO groups can be regarded as having a double bond
C=O group, as compared to a terminal C≡O, which is more like a
triple bond:
~ double bond
~ triple bond
M
M-C≡O
terminal carbonyl
(~ 1850-2125 cm-1)
C=O
M
bridging carbonyl
(~1700-1860 cm-1)
Bridging CO between 1700 and 2200 cm-1
the C=O group
in a bridging
carbonyl is more
like the C=O in
a ketone, which
typically has
υC=O = 1750 cm-1
Bridging versus terminal carbonyls in [Fe2(CO)9]
O
OC
OC
Fe
OC
CO
C
Fe
C
C O
CO
CO
O
terminal
carbonyls
bridging
carbonyls
Summary
1. As the CO bridges more metal centers its stretching
frequency drops – same for all p ligands
– More back donation
2. As the metal center becomes increasingly electron rich the stretching
frequency drops
Alkene ligands
Dewar-Chatt-Duncanson model
The greater the electron density
back-donated into the p* orbital on
the alkene, the greater the reduction
in the C=C bond order
Stability of alkene complexes also
depends on steric factors as well
An empirical ordering of relative
stability would be:
tetrasubstituted < trisubstituted <
trans-disubstituted < cisdisubstituted < monosubstituted <
ethylene
Alkyne ligands:
Similar to alkenes
Alkynes tend to be more
electropositive-bind more
tightly to a transition metal
than alkenes -alkynes will
often displace alkenes
Difference is 2 or 4 electron
donor
sigma-type fashion (A) as
we did for alkenes,
including a pi-backbond (B)
The orthogonal set can also
bind in a pi-type fashion
using an orthogonal metal
d-orbital (C)
The back-donation to the antibonding orbital (D) is a delta-bond-the
degree of overlap is quite small - contribution of D to the bonding of
alkynes is minimal
The net effect p-donation - alkynes are usually non-linear
in TM complexes
Resonance depict the bonding of an alkyne.
I is the metallacyclopropene resonance form
Support for this versus a simple two electron donor, II,
can be inferred from the C-C bond distance as well the RC-C-R angles
III generally does not contribute to the bonding of alkyne
complexes.
Ally ligands:
Allyl ligands are ambidentate ligands that can bind in both a
monohapto and trihapto form The trihapto form can be expressed as
a number of difference resonance forms as shown here for an
unsubstituted allyl ligand: Important applications
Dihydrogen Ligands:
Metal is more electropositive than hydrogen
Hydrogen acts as a two electron sigma donor to the metal center.
The complex is an arrested intermediate in the oxidative addition of dihydrogen
How does this affect the oxidation state of the
meta?
Dihydrogen complexes Bonding is “simple” a 3C-2electron bond.
H2 - neutral two electron sigma donor
One could also describe a back-donation of electrons from a filled metal
orbital to the sigma-* orbital on the dihydrogen
Electronic Attributes of Phosphines
Like that of carbonyls
As electron-withdrawing sigma-donating capacity decreases
At the same time, the energy of the p-acceptor (sigma-*) on phosphorous is
lowered in energy, providing an increase in backbonding ability.
Therefore, range of each capabilities –tuning rough ordering -CO stretching
frequency indicator- low CO stretching frequency- greater backbonding to M
Experiments such as this permit us to come up with the following empirical
ordering:
Cone Angle (Tolman)
Steric hindrance:
Phosphine
Ligand
Cone
Angle
A cone angle of 180 degrees effectively protects (or covers) one
half of the coordination sphere of
the metal complex
PH3
87o
PF3
104o
P(OMe)3
107o
PMe3
118o
PMe2Ph
122o
PEt3
132o
PPh3
145o
PCy3
170o
P(t-Bu)3
182o
P(mesityl)3
212o
You would expect a dissociation event
to occur first before any other reaction
-steric bulk (rate is first order
-increasing size)
This will also have an effect on
activity for catalysts
N.B. “flat” can slide past each other
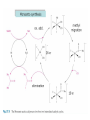
For example Wilkinson's catalyst
(more later)
Has a profound effect on the
reactivity!
Reaction chemistry of complexes
Three general forms:
1. Reactions involving the gain and loss of ligands
a. Ligand Dissoc. and Assoc. (Bala)
b. Oxidative Addition
c. Reductive Elimination
d. Nucleophillic displacement
2. Reactions involving modifications of the ligand
a. Insertion
b. Carbonyl insertion (alkyl migration)
c. Hydride elimination (equilibrium)
3. Catalytic processes by the complexes
Wilkinson, Monsanto
Carbon-carbon bond formation (Heck etc.)
a) Ligand dissociation/association (Bala)
• Electron count changes by -/+ 2
• No change in oxidation state
• Dissociation easiest if ligand stable on its own
(CO, olefin, phosphine, Cl-, ...)
• Steric factors important
M
Br
M
+ Br-
b) Oxidative Addition
Basic reaction:
LnM +
X
Y
X
LnM
Y
• Electron count changes by +/- 2
(assuming the reactant was not yet coordinated)
• Oxidation state changes by +/- 2
• Mechanism may be complicated The new M-X and M-Y bonds are
formed using:
• the electron pair of the X-Y bond
• one metal-centered lone pair
One reaction multiple mechanisms
Concerted addition, mostly with non-polar X-Y bonds
H2, silanes, alkanes, O2, ...
Arene C-H bonds more reactive than alkane C-H bonds (!)
LnM
+
X
Y
X
LnM
Y
A
Intermediate A is a s-complex
Reaction may stop here if metal-centered lone pairs
are not readily available
Final product expected to have cis X,Y groups
X
LnM
Y
Stepwise addition, with polar X-Y bonds
– HX, R3SnX, acyl and allyl halides, ...
– low-valent, electron-rich metal fragment (IrI, Pd(0), ...)
X
LnM
X Y
LnM X Y
LnM
Y
B
Metal initially acts as nucleophile
– Coordinative unsaturation less important
Ionic intermediate (B)
Final geometry (cis or trans) not easy to predict
Radical mechanism is also possible
Cis or trans products depends on the mechanism
H
H2
OC
Ir
Et3P
PEt3
H
Ir(III)
cis
Cl
H
OC
Et3P
PEt3
Ir Cl
HI
OC
Ir
Et3P
Ir(I)
Cl
PEt3
Ir(III)
I
cis
CH3
PEt3
CH3Br
OC
Ir
Cl
Br
trans
Et3P
Ir(III)
c) Reductive elimination
This is the reverse of oxidative addition - Expect cis elimination
Rate depends strongly on types of groups to be eliminated.
Usually easy for:
• H + alkyl / aryl / acyl
– H 1s orbital shape, c.f. insertion
• alkyl + acyl
– participation of acyl p-system
• SiR3 + alkyl etc
Often slow for:
• alkoxide + alkyl
• halide + alkyl
– thermodynamic reasons?
We will do a number of examples of this reaction
Relative rates of reductive elimination
L
CH3
Pd
L
L
-L
Ph3P
CH3
Pd
Ph3P
MePh2P
CH3
T(oC)
60
9.62 x 10-5
60
Ph
4.78 x 10-7
80
CH3
CH3
Pd
P
LPd(solv) + CH3
1.04 x 10-3
CH3
Pd
Ph
RE
CH3
MePh2P
P
solv
Rate Constant (s-1)
Complex
Ph
Pd
+ solv
CH3
CH3
Ph
CH3
Most crowded is the fastest reaction
CH3
Special case:
Nucleophilic Attack on a Coordinated CO acyl anion
Fisher carbene
This is Fischer carbene It has a metal carbon double bond
Such species can be made for relatively electronegative
metal centers N.B. mid to late TMs
Fischer carbenes are susceptible to nucleophilic attack at
the carbon
Fischer carbenes act effectively as σ donors and p acceptors
The empty antibonding M=C orbital is primarily on the carbon making it
susceptible to attack by nucleophiles
Other type is called a Shrock carbene (alkylidene)
Characteristic
Fischer-type
Schrock-type
Typical metal (Ox.
State)
Middle to late T.M.
Fe(0), Mo(0) Cr(0)
Early T.M.
Ti(IV), Ta(V)
Substituents
attached to carbene
carbon
At least one highly
electronegative
heteroatom
H or alkyl
Typical other
ligands
Good p acceptors
Good s and p
donors
Electron count
18
10-18
Nucleophilic displacement
Ligand displacement can be described as nucleophilic substitutions
O.M. complexes with negative charges can behave as nucleophiles
in displacement reactions Iron tetracarbonyl (anion) is very useful
O
R
R'
R'X
[Fe(CO)4]2-
RX
[ R
O
O2
Fe(CO)4]-
R
O
X2
H+
R H
CO
R
R
O
X
O
[ R
Fe(CO)4]-
H+
R
O
H
X
OH
Modifications of the ligand
a) Insertion reactions
Migratory insertion!
The ligands involved must be cis - Electron count changes by -/+ 2
No change in oxidation state
If at a metal centre you have a s-bound group (hydride, alkyl, aryl)
a ligand containing a p-system (olefin, alkyne, CO) the s-bound
group can migrate to the p-system
1. CO, RNC (isonitriles): 1,1-insertion
2. Olefins: 1,2-insertion, b-elimination
R
R
M
M
CO
1,1
O
R
M
M
R
1,2
1,1 Insertion
The s-bound group migrates to the p-system
if you only see the result, it looks like the p-system has inserted into the M-X
bond, hence the name insertion
To emphasize that it is actually (mostly) the X group that moves, we use the
term migratory insertion (Both possible tutorial)
The reverse of insertion is called elimination
Insertion reduces the electron count, elimination increases it
Neither insertion nor elimination causes a change in oxidation state
a- elimination can release the “new” substrate or compound
In a 1,1-insertion, metal and X group "move" to the same atom of the inserting
substrate.
The metal-bound substrate atom increases its valence
Me
M
M
CO
O
Me
Me
M
M
SO2
S Me
O O
CO, isonitriles (RNC) and SO2 often undergo 1,1-insertion
1,2 insertion (olefins)
Insertion of an olefin in a metal-alkyl bond produces a new alkyl
Thus, the reaction leads to oligomers or polymers of the olefin
• polyethene (polythene)
• polypropene
Standard Cossee mechanism
R
M
M
R
M
R
M
R
Why do olefins polymerise?
Driving force: conversion of a p-bond into a s-bond
One C=C bond: 150 kcal/mol
Two C-C bonds: 2´85 = 170 kcal/mol
Energy release: about 20 kcal per mole of monomer
(independent of mechanism)
Many polymerization mechanisms
Radical (ethene, dienes, styrene, acrylates)
Cationic (styrene, isobutene)
Anionic (styrene, dienes, acrylates)
Transition-metal catalyzed (a-olefins, dienes, styrene)
b Hydride elimination (usually by b hydrogens)
Many transition metal alkyls are unstable (the reverse of insertion)
the metal carbon bond is weak compared to a metal hydrogen
Bond Alkyl groups with β hydrogen tend to undergo β elimination
M -CH2-CH3 M - H + CH2=CH2
Two examples
A four-center transition state in which the hydride is transferred to the metal
An important prerequisite for beta-hydride elimination is the presence of an
open coordination site on the metal complex - no open site is available - displace
a ligand metal complex will usually have less than 18 electrons, otherwise a 20
electron olefin-hydride would be the immediate product.
To prevent beta-elimination from taking place, one can use alkyls that:
Do not contain beta-hydrogens
Are oriented so that the beta position can not access the metal center
Would give an unstable alkene as the product
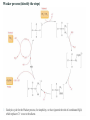
Catalysis (homogeneous)
Reduction of alkenes etc.
The size of the substrate has an effect on the rate of reaction
Same reaction different catalyst
Alternative starting material
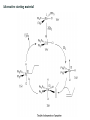
The Monsanto acetic acid process
Methanol - reacted with carbon monoxide in the presence of a catalyst to afford
acetic acid
Insertion of carbon monoxide into the C-O bond of methanol
The catalyst system - iodide and rhodium
Iodide promotes the conversion of methanol to methyl iodide,
Methyl iodide - the catalytic cycle begins:
1. Oxidative addition of methyl iodide to [Rh(CO)2I2]2. Coordination and insertion of CO - intermediate 18-electron acyl complex
3. Can then undergo reductive elimination to yield acetyl iodide and regenerate
our catalyst
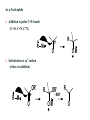
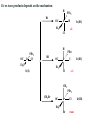
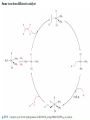
Catvia Process
Wacker process (identify the steps)
Identify the steps












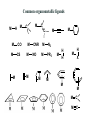


































































![[E]ven the most difficult problems in chemical experimentation can](http://s1.studyres.com/store/data/006510251_1-96239c5b6e245cee1be60ed8e0730b48-150x150.png)
