06 MC /08 MC /08 NMR
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
2.9 database - DrBravoChemistry
... why this compound can exist in two stereoisomeric forms. Type of isomerism ....................................................................................................... ...
... why this compound can exist in two stereoisomeric forms. Type of isomerism ....................................................................................................... ...
File - Dr KHALID SHADID
... The trigonal planar arrangement of groups around the carhonyl carbon atom means that the carbonyl carbon atom is relatively open to attack from above or below. The positive charge on the carbonyl carbon atom means that it is especially susceptible to attack by a nucleophile. The negative charge on ...
... The trigonal planar arrangement of groups around the carhonyl carbon atom means that the carbonyl carbon atom is relatively open to attack from above or below. The positive charge on the carbonyl carbon atom means that it is especially susceptible to attack by a nucleophile. The negative charge on ...
Enantioselective Henry Reactions under Dual Lewis Acid/Amine
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
... selectivity (entry 1), whereas increasing the ligand loading above 45 mol % did not improve the result. The quantity of iPr2EtN was crucial too. Lower loading or absence of iPr2EtN (entries 3 and 4) led to diminished yields and ee values. Interestingly, the absence of iPr2EtN could be partially comp ...
Carboxylates/esters vs ketones/aldehydes
... BH3 becomes B(OC2H5)3 by reacting with ethanol, then, when heated with water, becomes B(OH)3. The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Becau ...
... BH3 becomes B(OC2H5)3 by reacting with ethanol, then, when heated with water, becomes B(OH)3. The mechanism of the NaBH4 reduction in a protic solvent such as ethanol, methanol, and water is known to be quite complex since NaBH4 reacts with the solvent, e.g., NaBH4 + C2H5OH → NaBH3(OC2H5) + H2 Becau ...
PART 3 Principles and Applications of Organometallics in Catalysis
... of the uses of acetic anhydride are based on acetylation of alcohols and phenols. The side products from such processes such as acetic acid and methyl acetate can be recycled using a further adaptation of the Monsanto process. ...
... of the uses of acetic anhydride are based on acetylation of alcohols and phenols. The side products from such processes such as acetic acid and methyl acetate can be recycled using a further adaptation of the Monsanto process. ...
Lecture 15
... 1st Half Reaction: NAD+ + H+ + 2e- --> NADH E°’ = -0.320V 2nd Half Reaction (Note: Its reversed!): FADH2 --> FAD + 2H+ + 2eE°’ = +0.219V E°’= –0.320V + +0.219V ...
... 1st Half Reaction: NAD+ + H+ + 2e- --> NADH E°’ = -0.320V 2nd Half Reaction (Note: Its reversed!): FADH2 --> FAD + 2H+ + 2eE°’ = +0.219V E°’= –0.320V + +0.219V ...
A Diels-Alder Synthesis
... product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes more stable than the corresponding endo product and is due to a variety of steric and e ...
... product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes more stable than the corresponding endo product and is due to a variety of steric and e ...
Correlation Between Acidity, Basicity and Catalytic Performance of
... The sol-gel magnesia-alumina oxides show a surface area ranging from 237 to 323 m2/g with bimodel pore size distribution. Acid and basic sites coexist on the surface of the samples, depending on the molar ratio of MgO/Al2O3. When isopropanol was decomposed over the magnesia-alumina oxides, propene, ...
... The sol-gel magnesia-alumina oxides show a surface area ranging from 237 to 323 m2/g with bimodel pore size distribution. Acid and basic sites coexist on the surface of the samples, depending on the molar ratio of MgO/Al2O3. When isopropanol was decomposed over the magnesia-alumina oxides, propene, ...
Organic Chemistry Unit Test
... 1. We did two labs involving esters. In the first, we made a series of esters. In the second, we made two polyesters. Describe at least 3 ‘real world’ uses that you could imagine for the products of either of these labs based on your observations and data table. (3 marks) ...
... 1. We did two labs involving esters. In the first, we made a series of esters. In the second, we made two polyesters. Describe at least 3 ‘real world’ uses that you could imagine for the products of either of these labs based on your observations and data table. (3 marks) ...
11 - DR CLEM KUEK
... ideally consist of only the more volatile component. When the relatively pure component reaches the top of the fractionating column the temperature remains relatively stable. The material that condenses over a small temperature range near the boiling point of the substance of interest is collected. ...
... ideally consist of only the more volatile component. When the relatively pure component reaches the top of the fractionating column the temperature remains relatively stable. The material that condenses over a small temperature range near the boiling point of the substance of interest is collected. ...
Dissertation:
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
Amines - hisham
... 1. Zeisel’s method (for Alkoxy OR, and N-Alkyl): alkoxy group is treated with hydrogen iodide and the alkyl halide formed is further treated with silver nitrate to precipitate silver iodide, collected and weighed ...
... 1. Zeisel’s method (for Alkoxy OR, and N-Alkyl): alkoxy group is treated with hydrogen iodide and the alkyl halide formed is further treated with silver nitrate to precipitate silver iodide, collected and weighed ...
Novel amine-catalysed hydroalkoxylation reactions of
... acceptor (1.8 mmol) in alcohol (500 mL) in a 1 mL reaction vessel was added DBU (0.09 mmol) via a syringe. After stirring for the time indicated in Table 2 the solution was diluted with ether (30 mL) and washed with sat. NH4Cl (2 6 15 mL). The organic extracts were dried (MgSO4) and the solvent remo ...
... acceptor (1.8 mmol) in alcohol (500 mL) in a 1 mL reaction vessel was added DBU (0.09 mmol) via a syringe. After stirring for the time indicated in Table 2 the solution was diluted with ether (30 mL) and washed with sat. NH4Cl (2 6 15 mL). The organic extracts were dried (MgSO4) and the solvent remo ...
Problem Set Chapter 13 Solutions February 28, 2013 13.27 Draw
... 13.48 Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields a mixture of 1,2dimethylcyclohexene and isopropylidene cyclopentane. Propose a mechanism to account for the formation of both products. OH H+ ...
... 13.48 Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields a mixture of 1,2dimethylcyclohexene and isopropylidene cyclopentane. Propose a mechanism to account for the formation of both products. OH H+ ...
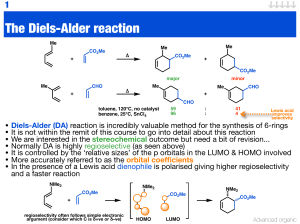
The Diels-Alder reaction
... • The fact the Diels-Alder reaction is mediated or catalysed by Lewis acids means enantioselective variants are readily carried out • The aluminium catalyst above has been utilised in enolate chemistry (aldol) reaction and is very effective in this Diels-Alder reaction ...
... • The fact the Diels-Alder reaction is mediated or catalysed by Lewis acids means enantioselective variants are readily carried out • The aluminium catalyst above has been utilised in enolate chemistry (aldol) reaction and is very effective in this Diels-Alder reaction ...
Reactions to functionalize benzene
... Due to the stability of aromatic system, addition reactions aren’t favored. Electrophilic aromatic substitution is the predominant reaction mechanism Hydrogens are easily replaced by electrophilic substituent groups H ...
... Due to the stability of aromatic system, addition reactions aren’t favored. Electrophilic aromatic substitution is the predominant reaction mechanism Hydrogens are easily replaced by electrophilic substituent groups H ...
Review Questions
... A 1.0 L reaction vessel is analyzed and found to contain 3.2 mol Cl2(g), 1.5 mol PCl3 (g) and 2.0 mol PCl5(g). Show that the reaction mixture has not yet reached equilibrium. 3. If 1.00 mol each of carbon dioxide and hydrogen is initially injected into a 10.0 L reaction chamber at 986oC, what would ...
... A 1.0 L reaction vessel is analyzed and found to contain 3.2 mol Cl2(g), 1.5 mol PCl3 (g) and 2.0 mol PCl5(g). Show that the reaction mixture has not yet reached equilibrium. 3. If 1.00 mol each of carbon dioxide and hydrogen is initially injected into a 10.0 L reaction chamber at 986oC, what would ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
CHEMISTRY 1000
... The alkoxide ion could alternately have been prepared by reacting the alcohol with sodium or potassium. This is usually done when the alcohol is also the solvent for the reaction. These reactions are analogous to the reactions between alkali metals and water that you studied in CHEM 1000. Do you rem ...
... The alkoxide ion could alternately have been prepared by reacting the alcohol with sodium or potassium. This is usually done when the alcohol is also the solvent for the reaction. These reactions are analogous to the reactions between alkali metals and water that you studied in CHEM 1000. Do you rem ...
TV RajanBabu Chemistry, 730 Autumn 1997
... Enolate generation (Z and E): Ireland model Examples of substituent, solvent, counter ion, base effects on enolate stereochemistry Boron enolates, including limitations Break down of Z-T transition states: two cases (explain with the aid of models): Aldol reactions of silyl enol ethers with Lewis ac ...
... Enolate generation (Z and E): Ireland model Examples of substituent, solvent, counter ion, base effects on enolate stereochemistry Boron enolates, including limitations Break down of Z-T transition states: two cases (explain with the aid of models): Aldol reactions of silyl enol ethers with Lewis ac ...
Answers
... This equilibrium is possible through the addition of acid to the electrophile, making it a better electrophile. This means it does not need as strong of a nucleophile to react. The reaction could be forced back to the left is water was added. 3. These reactive carboxylic acid derivatives will react ...
... This equilibrium is possible through the addition of acid to the electrophile, making it a better electrophile. This means it does not need as strong of a nucleophile to react. The reaction could be forced back to the left is water was added. 3. These reactive carboxylic acid derivatives will react ...
ppt
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
... The Wittig reaction is highly selective for ketones and aldehydes; esters, lactones, nitriles and amides will not react but are tolerated in the substrate. Acidic groups (alcohols, amine and carboxylic acids) are not tolerated. O O ...
Lecture 11a
... • Other considerations • Despite the addition of the catalyst, the rate of the reaction is still very low at room temperature • Reflux the mixture to increase the rate of the reaction ...
... • Other considerations • Despite the addition of the catalyst, the rate of the reaction is still very low at room temperature • Reflux the mixture to increase the rate of the reaction ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.