Discuss on Reactions of Alcohols
... decrease in acidity is due to two factors: an increase of electron density on the oxygen atom of the more highly‐substituted alcohol, and steric hindrance (because of the alkyl groups, which inhibit solvation of the resulting alkoxide ion). Both of these situations increase the activation energy for ...
... decrease in acidity is due to two factors: an increase of electron density on the oxygen atom of the more highly‐substituted alcohol, and steric hindrance (because of the alkyl groups, which inhibit solvation of the resulting alkoxide ion). Both of these situations increase the activation energy for ...
ORGANIC REACTIONS 14 APRIL 2015 Section A
... manipulated. Plastics cannot conduct electric current and are therefore electric insulators. They are not affected by weather or rust and are extremely versatile. ...
... manipulated. Plastics cannot conduct electric current and are therefore electric insulators. They are not affected by weather or rust and are extremely versatile. ...
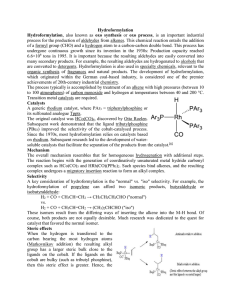
Hydroformylation Hydroformylation, also known as oxo synthesis or
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
Final Exam from 2006 - Department of Chemistry | Oregon State
... Drugs A and B below (Sertraline, the active ingredient in Zoloft) were synthesized in a lab. The amine group in compound A was neutralized with HCl to produce structure B, an ammonium salt. ...
... Drugs A and B below (Sertraline, the active ingredient in Zoloft) were synthesized in a lab. The amine group in compound A was neutralized with HCl to produce structure B, an ammonium salt. ...
Organic Chemistry
... • alkyl halides with an easily eliminated b hydrogen are rarely used because they undergo b-elimination to give alkenes. • OH groups and the C=O groups of aldehydes, ketones, and esters are unreactive under Heck conditions. ...
... • alkyl halides with an easily eliminated b hydrogen are rarely used because they undergo b-elimination to give alkenes. • OH groups and the C=O groups of aldehydes, ketones, and esters are unreactive under Heck conditions. ...
15 - MSU Chemistry
... has a plane of symmetry. The stereochemistry merely shows that the two OTs groups are on the same side of the molecule as drawn. Displacement with sulfur will occur with inversion ...
... has a plane of symmetry. The stereochemistry merely shows that the two OTs groups are on the same side of the molecule as drawn. Displacement with sulfur will occur with inversion ...
Topic 11 Organic Chemistry
... the following pairs of organic substances, whose boiling points are given: • ethane (184 K) and butane (273 K); • ethane ( 184 K) and bromoethane (311 K); • bromoethane (311 K) and ethanol (352 K). ...
... the following pairs of organic substances, whose boiling points are given: • ethane (184 K) and butane (273 K); • ethane ( 184 K) and bromoethane (311 K); • bromoethane (311 K) and ethanol (352 K). ...
CARBONYL COMPOUNDS ALDEHYDES AND KETONES
... difficult to reduce to aldehydes. This difficulty is circumvented by converting a carboxylic acid into the more reactive acid chloride, which can be readily reduced to an aldehyde. Lithium tri-t-butoxyaluminum hydride is a mild reducing agent that displaces chloride with hydride to produce an aldehy ...
... difficult to reduce to aldehydes. This difficulty is circumvented by converting a carboxylic acid into the more reactive acid chloride, which can be readily reduced to an aldehyde. Lithium tri-t-butoxyaluminum hydride is a mild reducing agent that displaces chloride with hydride to produce an aldehy ...
Preparation and Reaction of Carboxylic Acids - IDC
... carboxylation of organometallic intermediates. As shown in the following diagram, both methods begin with an organic halogen compound and the carboxyl group eventually replaces the halogen. Both methods require two steps, but are complementary in that the ...
... carboxylation of organometallic intermediates. As shown in the following diagram, both methods begin with an organic halogen compound and the carboxyl group eventually replaces the halogen. Both methods require two steps, but are complementary in that the ...
ALDOL CONDENSATION
... In the first step of the mechanism, an α‐proton is removed by a strong base, resulting in the formation of an enolate anion, which is made relatively stable by the delocalization of electrons. ¾ Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. ...
... In the first step of the mechanism, an α‐proton is removed by a strong base, resulting in the formation of an enolate anion, which is made relatively stable by the delocalization of electrons. ¾ Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. ...
Chapter 14 – Aldehydes and Ketones
... Common names occur frequently for aldehydes. These fall into two broad classes. The first type of name is derived from the name used for a common carboxylic acid. The name of the carboxylic acid typically comes from a Latin origin. For example, formaldehyde (CH2O) is derived from formic acid (HCO2H) ...
... Common names occur frequently for aldehydes. These fall into two broad classes. The first type of name is derived from the name used for a common carboxylic acid. The name of the carboxylic acid typically comes from a Latin origin. For example, formaldehyde (CH2O) is derived from formic acid (HCO2H) ...
Aldehid dan Keton
... • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
... • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
Unit 2 Review: Answers: Review for Organic Chemistry Unit Test 2
... reaction. Butanoic acid is a carboxylic acid so it can not be further oxidized. iv) chemical reactivity: butanal is an aldehyde so will not undergo esterification reactions with alcohols. Butanoic acid is a carboxylic acid so it will form esters with alcohols. The formation of an ester can be detect ...
... reaction. Butanoic acid is a carboxylic acid so it can not be further oxidized. iv) chemical reactivity: butanal is an aldehyde so will not undergo esterification reactions with alcohols. Butanoic acid is a carboxylic acid so it will form esters with alcohols. The formation of an ester can be detect ...
Chapter 20 Amines-part 2
... (H 3PO2) to replace the diazonium group with a hydrogen atom t This reaction can be used to remove an amino group that was ...
... (H 3PO2) to replace the diazonium group with a hydrogen atom t This reaction can be used to remove an amino group that was ...
10.5 Carbonyl Compounds (a) describe: (i) the
... (a) describe: (i) the formation of aldehydes and ketones from primary and secondary alcohols respectively using Cr2O72-/H+ (ii) the reduction of aldehydes and ketones e.g. using NaBH4 (b) describe the mechanism of nucleophilic addition reactions of hydrogen cyanide with aldehydes and ketones (c) des ...
... (a) describe: (i) the formation of aldehydes and ketones from primary and secondary alcohols respectively using Cr2O72-/H+ (ii) the reduction of aldehydes and ketones e.g. using NaBH4 (b) describe the mechanism of nucleophilic addition reactions of hydrogen cyanide with aldehydes and ketones (c) des ...
Kazzie`s Guide to Orgo 2
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...
... Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 21 ...
Notes 07 Organometallic Compounds
... Creation of new C-C bonds. ______________are best, otherwise an elimination reaction can occur. The R’ group in the halide can be ______________ The R group of the cuprate can be ______________ Although the mechanism looks like a _________ reaction, it is more complex and is not well understood. ...
... Creation of new C-C bonds. ______________are best, otherwise an elimination reaction can occur. The R’ group in the halide can be ______________ The R group of the cuprate can be ______________ Although the mechanism looks like a _________ reaction, it is more complex and is not well understood. ...
Biochemistry 462a - Enzymes Extra Questions
... twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming they have equal affinity for the enzyme, why would a noncompetitive inhibitor be a more effective drug than a competitive inhibitor? 7. Why ...
... twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming they have equal affinity for the enzyme, why would a noncompetitive inhibitor be a more effective drug than a competitive inhibitor? 7. Why ...
Document
... carboxylic acid. When catalyzed by a strong acid, usually sulfuric acid or p-toluenesulfonic acid, the reaction is called the Fischer esterification. The reaction mechanism is shown in Equations 2-6. Equation 2 shows the protonation of the acyl oxygen of the carboxylic acid. The protonation activate ...
... carboxylic acid. When catalyzed by a strong acid, usually sulfuric acid or p-toluenesulfonic acid, the reaction is called the Fischer esterification. The reaction mechanism is shown in Equations 2-6. Equation 2 shows the protonation of the acyl oxygen of the carboxylic acid. The protonation activate ...
ENZYMES - SELF STUDY QUESTIONS 1. A chemical reaction has a
... twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming they have equal affinity for the enzyme, why would a noncompetitive inhibitor be a more effective drug than a competitive inhibitor? 7. Why ...
... twice as fast for every 10o rise in temperature. This is true for enzymes up to a certain temperature at which point the reaction no longer occurs. 6. Assuming they have equal affinity for the enzyme, why would a noncompetitive inhibitor be a more effective drug than a competitive inhibitor? 7. Why ...
Ch 26 C-C bond formation
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
n - TU Chemnitz
... constants, and finally to understand the kinetics of the reactions. After that, different reactions like substitution, esterification, dehydration, oxidation of the hydroxy-group and 1,3-dipolar Cycloaddition, photolysis, Staudinger reaction of the azido-group should be performed. ...
... constants, and finally to understand the kinetics of the reactions. After that, different reactions like substitution, esterification, dehydration, oxidation of the hydroxy-group and 1,3-dipolar Cycloaddition, photolysis, Staudinger reaction of the azido-group should be performed. ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.