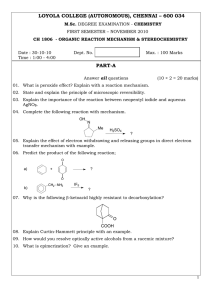
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 01. What is peroxide effect? Explain with a reaction mechanism. 02. State and explain the principle of microscopic reversibility. 03. Explain the importance of the reaction between neopentyl iodide and aqueous AgNO3. 04. Complete the following reaction with mechanism. OH ...
... 01. What is peroxide effect? Explain with a reaction mechanism. 02. State and explain the principle of microscopic reversibility. 03. Explain the importance of the reaction between neopentyl iodide and aqueous AgNO3. 04. Complete the following reaction with mechanism. OH ...
Chemistry Crunch #12.2: Organic Reactions KEY Why? Learning
... 3. Summarize. In a characteristic addition reaction: a) There will always be 2 reactant(s) and 1 product(s). b) The hydrocarbon reactant will always have a double or triple bond, or in other words, the hydrocarbon will be unsaturated. c) We learned how to classify many non-organic chemical reactions ...
... 3. Summarize. In a characteristic addition reaction: a) There will always be 2 reactant(s) and 1 product(s). b) The hydrocarbon reactant will always have a double or triple bond, or in other words, the hydrocarbon will be unsaturated. c) We learned how to classify many non-organic chemical reactions ...
Exam 2
... Exam Contents; The exam will be over chapter 9, 10 and 11. You can bring and use the Sn2 vs Sn1 vs E2 vs E1 summation sheets on the website for this exam. Most pertinent types of questions to study: In class #3, sample problems from chap 9 and 10 and practice exam #2 (see web site) are worth studyin ...
... Exam Contents; The exam will be over chapter 9, 10 and 11. You can bring and use the Sn2 vs Sn1 vs E2 vs E1 summation sheets on the website for this exam. Most pertinent types of questions to study: In class #3, sample problems from chap 9 and 10 and practice exam #2 (see web site) are worth studyin ...
This exam will consist of 30-35 multiple choice or short answer
... What is the product distribution? Why? How was this distribution determined? What is the electrophile? Reaction mechanism? Draw the intermediate, including all resonance structures. Is there a catalyst for this reaction? What is it? What did it do? How is GC used in this reaction? What did the chrom ...
... What is the product distribution? Why? How was this distribution determined? What is the electrophile? Reaction mechanism? Draw the intermediate, including all resonance structures. Is there a catalyst for this reaction? What is it? What did it do? How is GC used in this reaction? What did the chrom ...
Lecture12
... because it is much less expensive than palladium. Nickel-based catalysts tend to be less active and general, however. Nickel is better at activating aryl chlorides than palladium in some cases, however. Platinum has shown no activity in crosscoupling chemistry. ...
... because it is much less expensive than palladium. Nickel-based catalysts tend to be less active and general, however. Nickel is better at activating aryl chlorides than palladium in some cases, however. Platinum has shown no activity in crosscoupling chemistry. ...
Organometallic Reagents: Sources of Nucleophilic Carbon for
... If the carbonyl carbon of an aldehyde or ketone could be attacked by a nucleophilic carbon atom, R:-, instead of a hydride ion, both an alcohol and a new carbon-carbon bond would be formed. The class of compounds called organometallic reagents are strong bases and good nucleophiles and are useful in ...
... If the carbonyl carbon of an aldehyde or ketone could be attacked by a nucleophilic carbon atom, R:-, instead of a hydride ion, both an alcohol and a new carbon-carbon bond would be formed. The class of compounds called organometallic reagents are strong bases and good nucleophiles and are useful in ...
Alcohols
... Give a reagent that can be used in a test to distinguish between a ketone and an aldehyde. State what you would observe in the test. Reagent ............................................................................................................. Observation with ketone ......................... ...
... Give a reagent that can be used in a test to distinguish between a ketone and an aldehyde. State what you would observe in the test. Reagent ............................................................................................................. Observation with ketone ......................... ...
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015
... Formal product of the addition is the enol, which is often not isolated ...
... Formal product of the addition is the enol, which is often not isolated ...
• Pergamon
... MetaHoporphyrins have been used extensively as models for studying cytochrome P-450 monooxygenase activity.! Although these catalysts are known to carry out many useful reactions such as alkene epoxidation and hydrocarbon oxidation, their utility in organic synthesis has been limited. Recently we re ...
... MetaHoporphyrins have been used extensively as models for studying cytochrome P-450 monooxygenase activity.! Although these catalysts are known to carry out many useful reactions such as alkene epoxidation and hydrocarbon oxidation, their utility in organic synthesis has been limited. Recently we re ...
Exam 2 - Wake Forest University
... 11. (21 points, 3 each) Provide the major organic products for each of the following reactions. Include stereochemistry where necessary. If the predominant reaction pathway is an elimination pathway, list all the alkenes that will be formed and indicate which one will be formed in highest yield. OTs ...
... 11. (21 points, 3 each) Provide the major organic products for each of the following reactions. Include stereochemistry where necessary. If the predominant reaction pathway is an elimination pathway, list all the alkenes that will be formed and indicate which one will be formed in highest yield. OTs ...
2.10 Assessed Homework Task - A
... Give a reagent that can be used in a test to distinguish between a ketone and an aldehyde. State what you would observe in the test. Reagent ............................................................................................................. Observation with ketone ......................... ...
... Give a reagent that can be used in a test to distinguish between a ketone and an aldehyde. State what you would observe in the test. Reagent ............................................................................................................. Observation with ketone ......................... ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... The diazotization of aniline in moderately concentrated acid has been found to be second order in HNO2. Explain. How will you determine the configuration of a) () mandelic acid with respect to (+) lactic acid? b) () lactic acid with respect to (+) tartaric acid? How will you correlate the configur ...
... The diazotization of aniline in moderately concentrated acid has been found to be second order in HNO2. Explain. How will you determine the configuration of a) () mandelic acid with respect to (+) lactic acid? b) () lactic acid with respect to (+) tartaric acid? How will you correlate the configur ...
Carboxylic Acid Derivatives
... If it is A AC2 (with Nu), the intermediate and reactant (the protonated starting material) are both positively charged. Electronegative groups would destabilize both. However, the effect may be greater on the reactant because the positive charge is transferred to the carbonyl carbon by both an induc ...
... If it is A AC2 (with Nu), the intermediate and reactant (the protonated starting material) are both positively charged. Electronegative groups would destabilize both. However, the effect may be greater on the reactant because the positive charge is transferred to the carbonyl carbon by both an induc ...
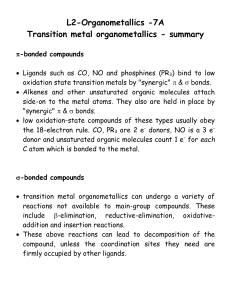
lect7
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
with answers
... reaction in which the number of gas-phase molecules decreases? The equilibrium is shifted to the right (fewer gas molecules = lowering of P) (1P) Which general Principle determines the effect of external influences (changes in temperature or pressure, removal of a reaction component etc) on a system ...
... reaction in which the number of gas-phase molecules decreases? The equilibrium is shifted to the right (fewer gas molecules = lowering of P) (1P) Which general Principle determines the effect of external influences (changes in temperature or pressure, removal of a reaction component etc) on a system ...
Transition Metal Chemistry 2 2011.12.2 Ⅰ Fundamental
... 2. Oxidative Addition and Reductive Elimination 3. Insertion and Elimination (De-insertion) 4. Reactions of Coordinated Ligands ...
... 2. Oxidative Addition and Reductive Elimination 3. Insertion and Elimination (De-insertion) 4. Reactions of Coordinated Ligands ...
Download
... 7. Methyl alcohol is industrially prepared from (a) CO + H 2 (b) C2 H 5 OH (c) CH 3 COCH 3 (d) CH 3 COOH 8. Benzyl alcohol is obtained from benzaldehyde by (a) Fittig's reaction (b)Cannizaro'sreaction (c) Kolbe'sreaction (d)Wurtz's reaction 9. Primary alcohols can be obtained from the reaction of th ...
... 7. Methyl alcohol is industrially prepared from (a) CO + H 2 (b) C2 H 5 OH (c) CH 3 COCH 3 (d) CH 3 COOH 8. Benzyl alcohol is obtained from benzaldehyde by (a) Fittig's reaction (b)Cannizaro'sreaction (c) Kolbe'sreaction (d)Wurtz's reaction 9. Primary alcohols can be obtained from the reaction of th ...
Stereoselective reactions of the carbonyl group
... • Underrated chiral auxiliary - easily introduced, performs many reactions & can be readily removed • One auxiliary can give either enantiomer of product • Selectivity dependent on whether reagent can chelate two groups Advanced organic ...
... • Underrated chiral auxiliary - easily introduced, performs many reactions & can be readily removed • One auxiliary can give either enantiomer of product • Selectivity dependent on whether reagent can chelate two groups Advanced organic ...
Worksheet 1 - Oregon State chemistry
... Did you identify the chemical formula of 2,2,4-trimethyl pentane to be C8H18? ...
... Did you identify the chemical formula of 2,2,4-trimethyl pentane to be C8H18? ...
L-13
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
... the reaction and led to the production of allylated product 3a in 80% yield (entry 2).[6] Strong Lewis acids such as AlCl3 or BF3・OEt2 were not effective for the allylation (entries 3 and 4), probably because these catalysts are not stable under protic conditions. Sc(OTf)3 only gave a low yield of 3 ...
Baylis–Hillman reaction

The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and an aldehyde, or generally a carbon electrophile. Employing a nucleophilic catalyst, such as tertiary amine and phosphine, this reaction provides a densely functionalized product (e.g. functionalized allyl alcohol in the case of aldehyde as the electrophile). This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction. It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman.DABCO is one of the most frequently used tertiary amine catalysts for this reaction. In addition, nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.MBH reaction has several advantages as a useful synthetic method: 1) It is an atom-economic coupling of easily prepared starting materials. 2) Reaction of a pro-chiral electrophile generates a chiral center, therefore an asymmetric synthesis is possible. 3) Reaction products usually contain multiple functionalities in a proximity so that a variety of further transformations are possible. 4) It can employ a nucleophilic organo-catalytic system without the use of heavy metal under mild conditions.Several reviews have been written.