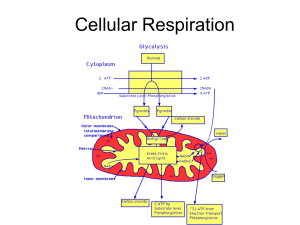
Cellular Respiration
... pretty much the opposite of each other! Photosynthesis • Plants • * use sunlight to make glucose • * take in carbon dioxide • * give off oxygen • *carbon dioxide + water + sunlight glucose + oxygen ...
... pretty much the opposite of each other! Photosynthesis • Plants • * use sunlight to make glucose • * take in carbon dioxide • * give off oxygen • *carbon dioxide + water + sunlight glucose + oxygen ...
... 19. The major reason for A pairing with U is: a) complementary hydrogen bonds. b) a purine-pyrimidine pair fits well in the double helix. c) efficient stacking of this arrangement of bases in the helix. d) recognition of non-’Watson-Crick’ hydrogen bonds by DNA polymerases 20. An expression vector o ...
Help Wanted
... fold it like a burrito. Place the burrito through the other sheet and then open the burrito. Fold the bound pages in half to form an eightpage book ...
... fold it like a burrito. Place the burrito through the other sheet and then open the burrito. Fold the bound pages in half to form an eightpage book ...
Thin-Layer Chromatography of Amino Acids
... 1. What are the four major types of macromolecules? 2. List the monomer for each of the four macromolecules. 3. How many essential amino acids make up proteins? 4. How is the structure of a protein similar to the structure of a paragraph? 5. What are the main functions of proteins in the body? ...
... 1. What are the four major types of macromolecules? 2. List the monomer for each of the four macromolecules. 3. How many essential amino acids make up proteins? 4. How is the structure of a protein similar to the structure of a paragraph? 5. What are the main functions of proteins in the body? ...
Document
... Residue - term used to refer to the amino acid once incorporated into a polypeptide Polypeptide - contain 10-100 residues Protein - contain more than 100 residues. Most peptides and proteins isolated from cells contain between 2 - 2000 residues. An average amino acid has a weight of 110, so protein ...
... Residue - term used to refer to the amino acid once incorporated into a polypeptide Polypeptide - contain 10-100 residues Protein - contain more than 100 residues. Most peptides and proteins isolated from cells contain between 2 - 2000 residues. An average amino acid has a weight of 110, so protein ...
Cellular Component of Blood
... bicarbonate, potassium, magnesium, phosphate, iron, calcium, copper, iodine, cobalt. Nutrients: specially from digested food e.g. monosaccharides (mainly glucose), amino acids, fatty acids, glycerol and vitamins. Organic waste materials: e.g. urea, uric acid, creatinine. Hormones. Enzymes, e.g. cert ...
... bicarbonate, potassium, magnesium, phosphate, iron, calcium, copper, iodine, cobalt. Nutrients: specially from digested food e.g. monosaccharides (mainly glucose), amino acids, fatty acids, glycerol and vitamins. Organic waste materials: e.g. urea, uric acid, creatinine. Hormones. Enzymes, e.g. cert ...
L16-Enzyme Structure
... Although only a small subset of the amino acids within an enzyme may engage the reactant(s), all enzyme constituents are needed for catalytic activity. Enormous molecule size generates: sufficient local-controlled flexibility precise three dimensional arrangements In spite of the tremendous stru ...
... Although only a small subset of the amino acids within an enzyme may engage the reactant(s), all enzyme constituents are needed for catalytic activity. Enormous molecule size generates: sufficient local-controlled flexibility precise three dimensional arrangements In spite of the tremendous stru ...
Chapter 10 Protein Synthesis
... A. 64 mRNA codons are possible 1. Four nucleotides in 3-letter combinations 2. 43 (4 cubed) B. There are only 20 amino acids 1. In some cases, several codons code for the same amino acid ...
... A. 64 mRNA codons are possible 1. Four nucleotides in 3-letter combinations 2. 43 (4 cubed) B. There are only 20 amino acids 1. In some cases, several codons code for the same amino acid ...
Metabolism_PartII Group work
... o The central metabolic pathways Glycolysis Pentose phosphate pathway Tricarboxylic acid cycle (TCA cycle) and transition step o Aerobic respiration o Anaerobic respiration o Fermentation Part B: Now label on each diagram how the harvested energy is stored during each catabolic process. AT ...
... o The central metabolic pathways Glycolysis Pentose phosphate pathway Tricarboxylic acid cycle (TCA cycle) and transition step o Aerobic respiration o Anaerobic respiration o Fermentation Part B: Now label on each diagram how the harvested energy is stored during each catabolic process. AT ...
Rna guided notes
... __RNA____ takes the DNA’s instructions out of the ___nucleus_______. RNA moves into the _cytoplasm______________ of the cell where there is room to make ___proteins (protein synthesis) _____________. Two locations of RNA: a. Nucleus b. Cytoplasm ...
... __RNA____ takes the DNA’s instructions out of the ___nucleus_______. RNA moves into the _cytoplasm______________ of the cell where there is room to make ___proteins (protein synthesis) _____________. Two locations of RNA: a. Nucleus b. Cytoplasm ...
Biology Quick Notes
... DNA is in the form of a twisted ladder aka DOUBLE HELIX RNA is single stranded Transcription- DNA is copied into mRNA Translation- mRNA is copied into tRNA o tRNA is attached to amino acid o amino acids make a chain… a chain of amino acids make a protein Mutations in DNA cause genetic variations o M ...
... DNA is in the form of a twisted ladder aka DOUBLE HELIX RNA is single stranded Transcription- DNA is copied into mRNA Translation- mRNA is copied into tRNA o tRNA is attached to amino acid o amino acids make a chain… a chain of amino acids make a protein Mutations in DNA cause genetic variations o M ...
1 PROBLEM SET 3 TCA cycle 1. To date this quarter you have
... and explain the circumstances that favor each. ...
... and explain the circumstances that favor each. ...
Amylase
... Digestive Enzymes: are used in the lumen of the GI tract to break down complex molecules into absorbable subunits Enzymes are biological catalysts which increase the rate of a chemical reaction without themselves becoming part of the product: ...
... Digestive Enzymes: are used in the lumen of the GI tract to break down complex molecules into absorbable subunits Enzymes are biological catalysts which increase the rate of a chemical reaction without themselves becoming part of the product: ...
GENETICS and the DNA code NOTES BACKGROUND DNA is the
... Translation takes place in the cytoplasm and is the process of creating a polypeptide from the information carried on the mRNA. The mRNA binds with a ribosome, which is the site where protein synthesis takes place. The mRNA moves through the ribosome three nitrogen bases at a time; these three nitro ...
... Translation takes place in the cytoplasm and is the process of creating a polypeptide from the information carried on the mRNA. The mRNA binds with a ribosome, which is the site where protein synthesis takes place. The mRNA moves through the ribosome three nitrogen bases at a time; these three nitro ...
Teaching Notes
... protease chains, while chain C is that of an inhibitor, designed based on a substrate (of the protease enzyme). Q2. Describe how the polymer chains are organized in the structure? Based on this description describe the function of HIV-1 Protease. A2: The 2 chains of HIV-1 Protease form a dimer with ...
... protease chains, while chain C is that of an inhibitor, designed based on a substrate (of the protease enzyme). Q2. Describe how the polymer chains are organized in the structure? Based on this description describe the function of HIV-1 Protease. A2: The 2 chains of HIV-1 Protease form a dimer with ...
Continuous flow-ultrasonic synergy in click reactions for
... network of interconnecting tubes. Although click reactions are traditionally conducted in batch process, the progress toward increased sustainability that requires novel approaches with reduced environmental impact opened up in recent years continuous-flow as a novel alternative to conventional batc ...
... network of interconnecting tubes. Although click reactions are traditionally conducted in batch process, the progress toward increased sustainability that requires novel approaches with reduced environmental impact opened up in recent years continuous-flow as a novel alternative to conventional batc ...
Chapter 26 - s3.amazonaws.com
... If ATP c.c. for a reaction in one direction differs from c.c. in the other, the reactions can form a substrate cycle • The point is not that ATP can be consumed by cycling • But rather that the difference in c.c. permits both reactions (pathways) to be thermodynamically favorable at all times • Allo ...
... If ATP c.c. for a reaction in one direction differs from c.c. in the other, the reactions can form a substrate cycle • The point is not that ATP can be consumed by cycling • But rather that the difference in c.c. permits both reactions (pathways) to be thermodynamically favorable at all times • Allo ...
A general trend for invertebrate mitochondrial genome evolution
... base and amino acids substitutions are equal. However, we also know that each organism will be affected by nature selection and a general trend for amino acids and nucleotides change should be existed. In 2005, Jordan et al reported the trends of amino acid changes were similar in 15 taxa representi ...
... base and amino acids substitutions are equal. However, we also know that each organism will be affected by nature selection and a general trend for amino acids and nucleotides change should be existed. In 2005, Jordan et al reported the trends of amino acid changes were similar in 15 taxa representi ...
Presentation Slides II - Vandiver, June 29, 2016
... beta pleated sheet. These structures are stabilized by hydrogen bonds. 3) Tertiary structure –the folding of the chains governed by hydrophobic or hydrophilic interactions. 4) Quaternary structure-- protein chains associating with other chains. The Star BioChem computer activity requires a working k ...
... beta pleated sheet. These structures are stabilized by hydrogen bonds. 3) Tertiary structure –the folding of the chains governed by hydrophobic or hydrophilic interactions. 4) Quaternary structure-- protein chains associating with other chains. The Star BioChem computer activity requires a working k ...
Weak interactions - Digilander
... Tertiary • Complete three-dimensional structure • Due to weak interactions between side (R) groups as well as covalent disulfide bonds Weak interactions Hydrogen bonds Electrostatic interactions (ionic bonds) Hydrophobic interactions Van der Waals interactions ...
... Tertiary • Complete three-dimensional structure • Due to weak interactions between side (R) groups as well as covalent disulfide bonds Weak interactions Hydrogen bonds Electrostatic interactions (ionic bonds) Hydrophobic interactions Van der Waals interactions ...
Ch. 5 Enzyme Review
... a. its substrate may not fit properly in the active site b. it will be missing one of its polypeptides c. the helical coil will be stretched out d. the product of the reaction will be a different molecule e. its substrate will bond covalently with the wrong part of the molecule 10. Why does heating ...
... a. its substrate may not fit properly in the active site b. it will be missing one of its polypeptides c. the helical coil will be stretched out d. the product of the reaction will be a different molecule e. its substrate will bond covalently with the wrong part of the molecule 10. Why does heating ...
Cell Respiration Test
... d. Free energy content 16. A noncompetitive inhibitor decreases the rate of an enzyme reaction by: a. Binding at the active site of an enzyme b. Changing the shape of an enzyme’s active site c. Changing the free energy change of the reaction d. Acting as a coenzyme for the reaction 17. HIV is the vi ...
... d. Free energy content 16. A noncompetitive inhibitor decreases the rate of an enzyme reaction by: a. Binding at the active site of an enzyme b. Changing the shape of an enzyme’s active site c. Changing the free energy change of the reaction d. Acting as a coenzyme for the reaction 17. HIV is the vi ...
bio12_sm_02_2
... 3. (a) The membranes are asymmetrical because the proteins and other components of one half of the lipid bilayer differ from those that make up the other half. (b) Membrane asymmetry reflects the differences in functions performed by each half of the membrane. 4. The phospholipids on the bilayer are ...
... 3. (a) The membranes are asymmetrical because the proteins and other components of one half of the lipid bilayer differ from those that make up the other half. (b) Membrane asymmetry reflects the differences in functions performed by each half of the membrane. 4. The phospholipids on the bilayer are ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.























