* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell Respiration Test
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
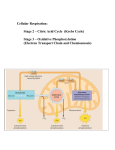
DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 1. Which of the following is true of metabolism in its entirety in all organisms? a. Metabolism depends on a constant supply of energy from food. b. Metabolism uses all of an organism’s resources. c. Metabolism consists of all the energy transformation reactions in an organism 2. Which of the following involves a decrease in entropy? a. Condensation reactions b. Reactions that separate monomers c. Depolymerization reactions d. Hydrolysis reactions 3. Which term most precisely describes the cellular processe3s of breaking down larger molecules into smaller ones? a. Catabolism b. Metabolism c. Anabolism d. Dehydration 4. Anabolic pathways _____________________: a. Are usually highly spontaneous chemical reactions b. Consume energy to build up polymers from monomers c. Release energy as they degrade polymers to monomers d. Consume energy to decrease the entropy of the organism and its environment 5. Which of the following is a statement of the first law of thermodynamics? a. Energy cannot be created or destroyed b. The entropy of the universe is decreasing c. The entropy of the universe is constant d. Energy cannot be transferred or transformed 6. For living organisms, which of the following is an important consequence of the first law of thermodynamics? a. The energy content of an organism is constant b. The organism ultimately must obtain all of the necessary energy for life from its environment c. Organisms grow by converting energy into organic matter 7. Which of the following statements is representative of the second law of thermodynamics?? a. Conversion of energy from one form to another is always accompanied by some gain of free energy b. Without an input of energy, organisms would tend toward decreasing entropy c. Cells require a constant input of energy to maintain their high level of organization DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 8. A system at equilibrium: a. Consumes energy at a steady rate b. Releases energy at a steady rate c. Has zero kinetic energy d. Can do no work 9. Which of the following is true for all exergonic reactions? a. The products have more total energy than the reactants b. The reaction proceeds with a net release of free energy c. The reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants d. A net input of energy from the surroundings is required for the reactions to proceed 10. A chemical reaction with a positive ∆ G is best described as: a. Endergonic b. Enthalpic c. Spontaneous d. Exergonic 11. Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as _____ is to ______. a. Exergonic; spontaneous b. Exergonic; endergonic c. Free energy; entropy d. Work; energy 12. Why is ATP an important molecule in metabolism: a. Its hydrolysis provides an input of free energy for exergonic reactions b. It provides energy coupling between exergonic and endergonic reactions c. Its terminal phosphate group contains a strong covalent bond that, when hydrolyzed, releases free energy d. Its terminal phosphate bond has higher energy than the other two phosphate bonds 13. Catabolic pathways _____________: a. Combine molecules into more energy-rich molecules b. Supply energy, primarily in the form of ATP, for cell’s work c. Are endergonic d. Are spontaneous and do not require enzyme catalysis DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 14. A number of systems for pumping ions across membranes are powered by ATP. Such ATPpowered pumps are often called ATPases, although they do not often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cystolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system known as the sarcoplasmic reticulum (SR). If a resting muscle cell’s cytosol has a free calcium ion concentration of 10-7 while the concentration in the SR is 10 -2 , then how is the ATPase acting? a. ATPase activity must be powering an inflow of calcium from the outside of the cell into the SR. b. ATPase activity must be transferring Potassium ions to the SR to enable this to occur c. ATPase activity must be pumping calcium from the cytosol to the SR against the concentration gradient. d. ATPase activity must be opening a channel for the calcium ions to diffuse back into the SR along the concentration gradient 15. Reactants capable of interacting to form products in a chemical reaction must first overcome a thermodynamic barrier known as the reaction’s: a. Entropy b. Activation energy c. Equilibrium point d. Free energy content 16. A noncompetitive inhibitor decreases the rate of an enzyme reaction by: a. Binding at the active site of an enzyme b. Changing the shape of an enzyme’s active site c. Changing the free energy change of the reaction d. Acting as a coenzyme for the reaction 17. HIV is the virus that causes AIDS. In the mid 1990s, researchers discovered an enzyme in HIV called protease. One the enzyme’s structure was known, researchers began looking for drugs that would fit into the active site and block it. If this strategy for stopping HIV infections were successful, it would be an example of what phenomenon? a. Vaccination b. Denaturation c. Allosteric regulation d. Competitive inhibition 18. Allosteric enzyme regulation is usually associated with: a. Feedback inhibition b. Activating activity c. An enzyme with more than one subunit d. The need for cofactors DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 19. Substrate-level phosphorylation occurs: a. In glycolysis b. In the citric acid cycle c. In both glycolysis and the citric acid cycle 20. The molecule that functions as a reducing agent (electron donor) in a redox or oxidationreduction reaction: a. Gains electron and gains potential energy b. Loses electrons and loses potential energy c. Gains electrons and loses potential energy d. Loses electrons and gains potential energy 21. When a molecule of NAD+ gains a hydrogen atom (not a proton), the molecule becomes: a. Dehydrogenated b. Oxidized c. Reduced d. Redoxed 22. Carbohydrates and fats are considered high-energy foods because they have a lot of: a. Have a lot of oxygen atoms b. Have no nitrogen in their makeup c. Have a lot of electrons associated with hydrogen d. Are easily reduced 23. Starting with one molecule of glucose, the energy-containing products of glycolysis are: a. 2 NAD+, 2 pyruvate, and 2 ATP b. 2 NADH, 2 pyruvate, and 2 ATP c. 2 FADH2, 2 pyruvate, and 4 ATP d. 6 CO2, 2 pyruvate, and 2 ATP 24. Most of the CO2 from the catabolism of glucose is released during: a. Glycolysis b. Electron transport c. Chemiosmosis d. The citric acid cycle 25. Carbon dioxide (CO2) is released during which of the following stages of cellular respiration? a. Glycolysis and the oxidation of pyruvate to acetyl CoA b. Oxidation of pyruvate to acetyl CoA and the citric acid cycle c. Oxidative phosphorylation and fermentation d. Fermentation and glycolysis DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 26. Which of the following events takes place in the electron transport chain? a. The breakdown of glucose into two pyruvate molecules b. The breakdown of an acetyl group to carbon dioxide c. The extraction of energy from high-energy electrons remaining from glycolysis and the citric acid cycle d. Substrate-level phosphorylation 27. The electron-transport chain: a. Is a series of redox reactions b. Is a series of substitution reactions c. Is driven by ATP consumption d. Takes place in the cytoplasm of prokaryotic cells 28. Energy released by the electron transport chain is used to pump H+ into which location in eukaryotic cells? a. Cell wall b. Mitochondrial matrix c. Mitochondrial intermembrane space d. Mitochondrial Matrix 29. When hydrogen ions are pumped from the mitochondrial matrix across the inner membrane and into the intermembrane space, the result is the: a. Formation of ATP b. Reduction of NAD+ c. Forcing of H+ ions through ATP Synthase d. Lowering of pH in the mitochondrial matrix 30. In chemiosmosis, what is the most direct source of energy that is used to convert ADP to ATP? a. Energy released as electrons flows through the electron transport system b. Energy released from substrate-level phosphorylation c. Energy released from movement of protons through ATP synthase, down their electrochemical gradient d. No external source of energy is required because the reaction is exergonic 31. Approximately how many molecules of ATP are produced from the complete oxidation of one molecule of glucose (C6H12O6) in aerobic cellular respiration? a. 2 b. 4 c. 18-24 d. 30-32 DO NOT WRITE ON MULTIPLE CHOICE AP BIO DO NOT WRITE ON MULTIPLE CHOICE Chapter 8/9 Test - Metabolism and Cellular Respiration 32. The synthesis of ATP by oxidative phosphorylation, using energy released by movement of protons across the membrane, down their electrochemical gradient, is an example of : a. Active transport b. An endergonic reaction coupled to an exergonic reaction c. A reaction with a positive Delta G d. Allosteric regulation 33. Which of the following normally occurs regardless of whether or not oxygen is present? a. Glycolysis b. Fermentation c. Citric acid cycle d. Oxidative phosphorylation 34. In the absence of oxygen, yeast cells can obtain energy by fermentation, resulting in the production of: a. ATP, CO2, and ethanol (ethyl alcohol) b. ATP, CO2, and lactate c. ATP, NADH, and pyruvate d. ATP, pyruvate, and acetyl CoA 35. Fatty acids usually have an even number of carbons in their structures. They are catabolized by a process called beta-oxidation. The end products of the metabolic pathways are acetyl groups of acetyl CoA molecules. The acetyl groups: a. Directly enter the electron transport chain b. Directly enter the energy-yielding stages of glycolysis c. Are directly decarboxylated by pyruvate dehydrogenase d. Directly enter the citric acid cycle Standards: 21st CENTURY LIFE AND CAREERS, 9.3O1 Grade 12 CPI 5 SCIENCE, 1.HS-73 Grade 12 CPI DCI-2 SCIENCE, 1.HS-73 Grade 12 CPI SEP-1