Bioener Notes - MacsScienceSpace
... In one minute a working muscle cell uses 10,000,000 ATP molecules. That is the cell's entire supply, so ADP must be recycled into ATP. Produce 125lbs of ATP per day. Blue Whale makes 5 tons/day. Even resting in bed, you use 20 kg of ATP every 24 hours! ...
... In one minute a working muscle cell uses 10,000,000 ATP molecules. That is the cell's entire supply, so ADP must be recycled into ATP. Produce 125lbs of ATP per day. Blue Whale makes 5 tons/day. Even resting in bed, you use 20 kg of ATP every 24 hours! ...
4.2 Respiration – Page 1 S. Preston 1 From the
... 5. The link reaction involves the conversion of pyruvate to acetate as a result of the loss of carbon dioxide followed by the removal of hydrogen by the reduction of NAD (oxidative decarboxylation); the acetyl then combines with co-enzyme A. The link reaction takes place in the matrix of the mitocho ...
... 5. The link reaction involves the conversion of pyruvate to acetate as a result of the loss of carbon dioxide followed by the removal of hydrogen by the reduction of NAD (oxidative decarboxylation); the acetyl then combines with co-enzyme A. The link reaction takes place in the matrix of the mitocho ...
... 8. (10 pts) Please do one of the following three questions. Please indicate your choice when answering the question. Choice A: A glutamic acid residue must be deprotonated for a protein to have biological function. The pKa of this glutamic acid residue is 5.0. Draw a graph that shows the activity of ...
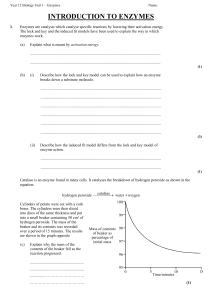
introduction to enzymes
... Enzymes are catalysts which catalyse specific reactions by lowering their activation energy. The lock and key and the induced fit models have been used to explain the way in which enzymes work. (a) ...
... Enzymes are catalysts which catalyse specific reactions by lowering their activation energy. The lock and key and the induced fit models have been used to explain the way in which enzymes work. (a) ...
Diversity in biological molecules
... the selection can hardly be called ‘natural’ since it has been imposed by a human experimenter. Although significant progress is being made, it remains very difficult to identify the key differences between proteins that have adaptive significance in eukaryotic organisms in the natural environment. ...
... the selection can hardly be called ‘natural’ since it has been imposed by a human experimenter. Although significant progress is being made, it remains very difficult to identify the key differences between proteins that have adaptive significance in eukaryotic organisms in the natural environment. ...
Secondary Structure - 3D Molecular Designs
... Fold the beta sheet and alpha helix into the final tertiary structure of the zinc finger. In its final tertiary structure, the seven side chains will be positioned such that: • The two cysteine and two histidine side chains will be oriented to simultaneously bind to a single zinc atom (not included ...
... Fold the beta sheet and alpha helix into the final tertiary structure of the zinc finger. In its final tertiary structure, the seven side chains will be positioned such that: • The two cysteine and two histidine side chains will be oriented to simultaneously bind to a single zinc atom (not included ...
Biochemistry-Review of the Basics
... Enzymes work best at an ideal temp and pH The speed of the reaction also depends on the amount of enzyme or substrate ...
... Enzymes work best at an ideal temp and pH The speed of the reaction also depends on the amount of enzyme or substrate ...
Cytochromes
... ► Chemiosmotic Potential or Proton-Motive Force (PMF) The electrochemical potential difference between the two sides of the IMM, that engage in active transport of Protons is called Proton-Motive Force (PMF). ► Proton motive force is the energy of the proton ...
... ► Chemiosmotic Potential or Proton-Motive Force (PMF) The electrochemical potential difference between the two sides of the IMM, that engage in active transport of Protons is called Proton-Motive Force (PMF). ► Proton motive force is the energy of the proton ...
Cellular Respiration
... • All energy is stored in the bonds of compounds —breaking the bond releases the energy • When the cell has energy to store it adds a phosphate group to ADP - producing ATP • When the cell needs energy for life processes, it breaks the bond holding the phosphate group and changes ATP back to ADP • A ...
... • All energy is stored in the bonds of compounds —breaking the bond releases the energy • When the cell has energy to store it adds a phosphate group to ADP - producing ATP • When the cell needs energy for life processes, it breaks the bond holding the phosphate group and changes ATP back to ADP • A ...
part c – can peroxidase react with other substances?
... bonding behavior. Each carbon atom can share pairs of electrons with as many as 4 other atoms. This provides a stable covalent bond since the electrons are shared equally. The carbons can either form a line that we call a ‘backbone’ or a ring structure. Other atoms such as hydrogen, oxygen, and othe ...
... bonding behavior. Each carbon atom can share pairs of electrons with as many as 4 other atoms. This provides a stable covalent bond since the electrons are shared equally. The carbons can either form a line that we call a ‘backbone’ or a ring structure. Other atoms such as hydrogen, oxygen, and othe ...
Chapter 7: PowerPoint
... 1. aerobic respiration – occurs when oxygen is available as the final electron acceptor 2. fermentation – occurs when oxygen is not available; an organic molecule is the final electron acceptor ...
... 1. aerobic respiration – occurs when oxygen is available as the final electron acceptor 2. fermentation – occurs when oxygen is not available; an organic molecule is the final electron acceptor ...
Basics of Molecular Biology
... https://ocw.mit.edu/courses/biology/7-343-network-medicine-using-systems-biology-and-signaling- ...
... https://ocw.mit.edu/courses/biology/7-343-network-medicine-using-systems-biology-and-signaling- ...
Cell Respiration
... – Energy is invested as 2 ATPs are hydrolyzed during steps 1 and 3 – One 6 carbon carbohydrate intermediate is split (lysis) into two 3 carbon carbohydrate intermediates during step 4 – each of the 3 carbon carbohydrate intermediates molecules lose a H (becoming oxidized) which is accepted by a coen ...
... – Energy is invested as 2 ATPs are hydrolyzed during steps 1 and 3 – One 6 carbon carbohydrate intermediate is split (lysis) into two 3 carbon carbohydrate intermediates during step 4 – each of the 3 carbon carbohydrate intermediates molecules lose a H (becoming oxidized) which is accepted by a coen ...
2002
... 54. Which term most precisely describes the cellular process of breaking down larger molecules into smaller molecules is (A) catalysis (B) metabolism (C) anabolism (D) dehydration (E) catabolism 55. The mathematical expression for the change in free energy in a system is G = H - TS. Which of the ...
... 54. Which term most precisely describes the cellular process of breaking down larger molecules into smaller molecules is (A) catalysis (B) metabolism (C) anabolism (D) dehydration (E) catabolism 55. The mathematical expression for the change in free energy in a system is G = H - TS. Which of the ...
Thermodynamics of Protein Folding
... – Large stabilization factors, large destabilization factors, but small difference between them – Use RNase T1 as a model for study (because structure is well known and many mutants have been studied) ...
... – Large stabilization factors, large destabilization factors, but small difference between them – Use RNase T1 as a model for study (because structure is well known and many mutants have been studied) ...
3.5 Transcription and translation – summary of
... 64 different codons; each codes for the addition of an amino acid to a growing polypeptide chain; the genetic code is degenerate; meaning more than one codon can code for a particular amino acid; the genetic code is universal; meaning it is the same in almost all organisms; (AUG is the) start codon; ...
... 64 different codons; each codes for the addition of an amino acid to a growing polypeptide chain; the genetic code is degenerate; meaning more than one codon can code for a particular amino acid; the genetic code is universal; meaning it is the same in almost all organisms; (AUG is the) start codon; ...
Chapter 2 Review
... a pure substance made of one type of atom _____ is any substance that has mass and volume bond formed by sharing a pair of electrons reaction that gives off free energy substances composed of two or more different atoms the rule of eight is also called the _____ rule ...
... a pure substance made of one type of atom _____ is any substance that has mass and volume bond formed by sharing a pair of electrons reaction that gives off free energy substances composed of two or more different atoms the rule of eight is also called the _____ rule ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.