7A SCIENCE FINAL REVIEW - MERRICK 7th SCIENCE REVIEW
... ___ Describe the difference between atoms and molecules. ___ Define elements, compounds, and mixtures. ___ Recognize elements from compounds if given the chemical symbol or a model. ___ Describe the difference between a chemical and physical property of matter, give examples of each. ___ Describe th ...
... ___ Describe the difference between atoms and molecules. ___ Define elements, compounds, and mixtures. ___ Recognize elements from compounds if given the chemical symbol or a model. ___ Describe the difference between a chemical and physical property of matter, give examples of each. ___ Describe th ...
Normal version 1.00
... and lithium ion battery electrodes and strain control in strained more difficult to make mechanical measurements of such small silicon/silicon germanium substrates for high speed microelectronic volumes of material. Nanoindentation is one of the few methods devices. available that can measure the e ...
... and lithium ion battery electrodes and strain control in strained more difficult to make mechanical measurements of such small silicon/silicon germanium substrates for high speed microelectronic volumes of material. Nanoindentation is one of the few methods devices. available that can measure the e ...
Unit 5 Chemical Properties and Changes Video Notes A ______ is a
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
Cluster Fragmentation and Catalysis
... and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catalyze reactions that would normally not occur under normal conditions. ...
... and condensed phases; they can be used as tools for selective microsolvation, or they can be a distinct class of materials with unique properties. Clusters, upon impact with a rigid surface at supersonic velocities, can also catalyze reactions that would normally not occur under normal conditions. ...
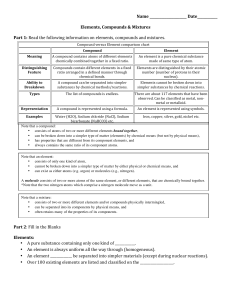
Compound vs Element chart
... Compounds: • A pure substance containing two or more kinds of _______________. • The atoms are _________________ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. • A compound is always homogeneous (uniform). • Compounds ________ ...
... Compounds: • A pure substance containing two or more kinds of _______________. • The atoms are _________________ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. • A compound is always homogeneous (uniform). • Compounds ________ ...
PowerPoint
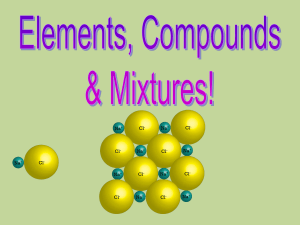
... All one kind of atom. Compounds are substances that can be broken down by chemical methods • When they are broken down, the pieces have completely different properties than the compound. • Made of molecules- two or more atoms ...
... All one kind of atom. Compounds are substances that can be broken down by chemical methods • When they are broken down, the pieces have completely different properties than the compound. • Made of molecules- two or more atoms ...
can be determined without changing the identity of matter
... - Cannot be broken down into simpler substances using physical or chemical means - Elements are the building blocks of chemistry! They are the simple things ...
... - Cannot be broken down into simpler substances using physical or chemical means - Elements are the building blocks of chemistry! They are the simple things ...
Unit 4 Evolution
... Let’s review yesterday’s activities and begin a couple of review activities over energy and properties of matter. ...
... Let’s review yesterday’s activities and begin a couple of review activities over energy and properties of matter. ...
PS7aChemistryReviewRevised
... What other categories are there? What is a mixture? Elements cannot be separated into anything simpler by human means – only smaller particles with the same characteristics! Compounds and mixtures can be separated into simpler components. Mixtures show the characteristics of the substances that the ...
... What other categories are there? What is a mixture? Elements cannot be separated into anything simpler by human means – only smaller particles with the same characteristics! Compounds and mixtures can be separated into simpler components. Mixtures show the characteristics of the substances that the ...
Perspective for Journal of Pharmaceutical Science
... Pharmaceutical co-crystals Pharmaceutical co-crystals can be defined as crystalline materials comprised of an active pharmaceutical ingredient (API) and one or more unique co-crystal formers, which are solids at room temperature. Co-crystals can be constructed through several types of interaction, i ...
... Pharmaceutical co-crystals Pharmaceutical co-crystals can be defined as crystalline materials comprised of an active pharmaceutical ingredient (API) and one or more unique co-crystal formers, which are solids at room temperature. Co-crystals can be constructed through several types of interaction, i ...
Chemical Bonding Lab
... Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two basic types—ionic and covalent. Ionic bonds result when one or more electrons from one atom or group of atoms is transferred to another atom. Positive and negative ions are created through t ...
... Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two basic types—ionic and covalent. Ionic bonds result when one or more electrons from one atom or group of atoms is transferred to another atom. Positive and negative ions are created through t ...
www.ualberta.ca - University of Alberta
... polymorphism and solvate formation, thus requiring the same form identification studies as are needed for a neutral compound. A remarkable example of co-optimization of properties is indinavir (HIV protease inhibitor), which is marketed as the sulfate salt ethanol solvate (24,25) The crystalline fre ...
... polymorphism and solvate formation, thus requiring the same form identification studies as are needed for a neutral compound. A remarkable example of co-optimization of properties is indinavir (HIV protease inhibitor), which is marketed as the sulfate salt ethanol solvate (24,25) The crystalline fre ...
Slide 1
... •Property of matter that describes a substances ability to participate in chemical reactions. (Change into new matter.) –Flammability •ability to burn •Burning wood creates ash and smoke ...
... •Property of matter that describes a substances ability to participate in chemical reactions. (Change into new matter.) –Flammability •ability to burn •Burning wood creates ash and smoke ...
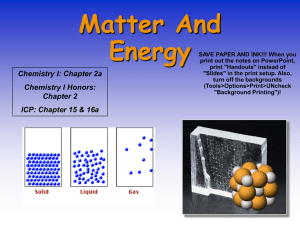
What are the four states of matter?
... Mass: amount of matter in an object Volume: amount of space an object occupies ...
... Mass: amount of matter in an object Volume: amount of space an object occupies ...
LIST OF TOPICS COVERED DURING THIS COURSE
... The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homework questions, lab questions, assignments, ...
... The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homework questions, lab questions, assignments, ...
Unit 01 Qual Chem
... Physical Change = a change that does not alter the identity of a substance (shape, size, state) Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
... Physical Change = a change that does not alter the identity of a substance (shape, size, state) Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
What is a mixture?
... Identifying Elements • Elements are categorized by unique properties on the Periodic Table. • They are arranged in order by their number of protons. (More on this later!) • Each element has unique properties like melting point, boiling point, and whether it is metal, nonmetal or metalloid. ...
... Identifying Elements • Elements are categorized by unique properties on the Periodic Table. • They are arranged in order by their number of protons. (More on this later!) • Each element has unique properties like melting point, boiling point, and whether it is metal, nonmetal or metalloid. ...
NAME
... Describe the process of distillation. Does the separation of the components of a mixture by distillation represent a chemical or a physical change? ...
... Describe the process of distillation. Does the separation of the components of a mixture by distillation represent a chemical or a physical change? ...
Chemistry Unit Study Guide Key
... laws says that no matter can be created or destroyed. Therefore, each side of the equation must be the same. ...
... laws says that no matter can be created or destroyed. Therefore, each side of the equation must be the same. ...
Matter in Chemistry
... Boiling the egg: when you use high heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg w ...
... Boiling the egg: when you use high heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white (it won't hurt you to eat it, and the egg w ...
Characteristic Properties Non-Characteristic Properties
... Physical Properties • These can be observed or measured without changing the make-up of the matter of the object • These properties can be used to describe the object • Two categories: non-characteristic and characteristic properties ...
... Physical Properties • These can be observed or measured without changing the make-up of the matter of the object • These properties can be used to describe the object • Two categories: non-characteristic and characteristic properties ...